Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D
- PMID: 20427486
- PMCID: PMC2928899
- DOI: 10.1210/jc.2010-0195
Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D
Abstract
Background: Serum 25-hydroxyvitamin D (25OHD) is a key factor in determining monocyte induction of the antimicrobial protein cathelicidin, which requires intracrine conversion of 25OHD to 1,25-dihydroxyvitamin D [1,25(OH)(2)D]. Both vitamin D metabolites circulate bound to vitamin D-binding protein (DBP), but the effect of this on induction of monocyte cathelicidin remains unclear.
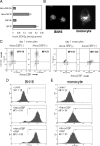
Methods: Human monocytes were cultured in medium containing 1) serum from DBP knockout (DBP(-/-)) or DBP(+/-) mice, 2) serum-free defined supplement reconstituted with DBP or albumin (control), and 3) human serum with different DBP [group-specific component [Gc]] genotypes with varying affinities for vitamin D metabolites. In each case, response to added 1,25(OH)(2)D(3) or 25OHD(3) was determined by measuring expression of mRNA for cathelicidin and 24-hydroxylase. Monocyte internalization of DBP was assessed by fluorescent tagging followed by microscopic and flow cytometric analysis of tagged DBP.
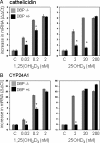
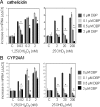
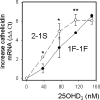
Results: Monocytes cultured in DBP(-/-) serum showed more potent induction of cathelicidin by 25OHD(3) or 1,25(OH)(2)D(3) when compared with DBP(+/-) serum. Likewise, DBP added to serum-free medium attenuated 25OHD(3)/1,25(OH)(2)D(3) responses. Fluorescently tagged DBP showed low-level uptake by monocytes, but this did not appear to involve a megalin-mediated mechanism. Human serum containing low-affinity Gc2-1S or Gc2-2, respectively, supported 2.75-fold (P = 0.003) and 2.43-fold (P = 0.016) higher induction of cathelicidin by 25OHD relative to cells cultured with high affinity Gc1F-1F.
Conclusion: These data indicate that DBP plays a pivotal role in regulating the bioavailablity of 25OHD to monocytes. Vitamin D-dependent antimicrobial responses are therefore likely to be strongly influenced by DBP polymorphisms.
Figures

References
-
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 - PubMed
-
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sørensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ 2007 IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 178:7190–7198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

