Role of interferon antagonist activity of rabies virus phosphoprotein in viral pathogenicity
- PMID: 20427527
- PMCID: PMC2903245
- DOI: 10.1128/JVI.00011-10
Role of interferon antagonist activity of rabies virus phosphoprotein in viral pathogenicity
Abstract
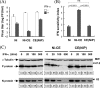
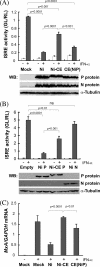
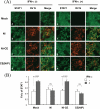
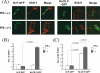
The fixed rabies virus (RV) strain Nishigahara kills adult mice after intracerebral inoculation, whereas the chicken embryo fibroblast cell-adapted strain Ni-CE causes nonlethal infection in adult mice. We previously reported that the chimeric CE(NiP) strain, which has the phosphoprotein (P protein) gene from the Nishigahara strain in the genetic background of the Ni-CE strain, causes lethal infection in adult mice, indicating that the P gene is responsible for the different pathogenicities of the Nishigahara and Ni-CE strains. Previous studies demonstrated that RV P protein binds to the interferon (IFN)-activated transcription factor STAT1 and blocks IFN signaling by preventing its translocation to the nucleus. In this study, we examine the molecular mechanism by which RV P protein determines viral pathogenicity by comparing the IFN antagonist activities of the Nishigahara and Ni-CE P proteins. The results, obtained from both RV-infected cells and cells transfected to express P protein only, show that Ni-CE P protein is significantly impaired for its capacity to block IFN-activated STAT1 nuclear translocation and, consequently, inhibits IFN signaling less efficiently than Nishigahara P protein. Further, it was demonstrated that a defect in the nuclear export of Ni-CE P protein correlates with a defect in its ability to cause the mislocalization of STAT1. These data provide the first evidence that the capacity of the RV P protein to inhibit STAT1 nuclear translocation and IFN signaling correlates with the viral pathogenicity.
Figures



References
-
- Chelbi-Alix, M. K., A. Vidy, J. El Bougrini, and D. Blondel. 2006. Rabies viral mechanisms to escape the IFN system: the viral protein P interferes with IRF-3, Stat1, and PML nuclear bodies. J. Interferon Cytokine Res. 26:271-280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

