Human immunodeficiency virus type 1 nucleocapsid p1 confers ESCRT pathway dependence
- PMID: 20427536
- PMCID: PMC2903293
- DOI: 10.1128/JVI.00035-10
Human immunodeficiency virus type 1 nucleocapsid p1 confers ESCRT pathway dependence
Abstract
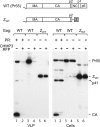
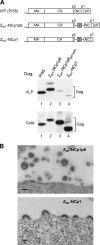
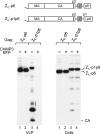
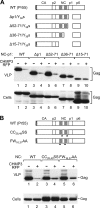
To facilitate the release of infectious progeny virions, human immunodeficiency virus type 1 (HIV-1) exploits the Endosomal Sorting Complex Required for Transport (ESCRT) pathway by engaging Tsg101 and ALIX through late assembly (L) domains in the C-terminal p6 domain of Gag. However, the L domains in p6 are known to be dispensable for efficient particle production by certain HIV-1 Gag constructs that have the nucleocapsid (NC) domain replaced by a foreign dimerization domain to substitute for the assembly function of NC. We now show that one such L domain-independent HIV-1 Gag construct (termed Z(WT)) that has NC-p1-p6 replaced by a leucine zipper domain is resistant to dominant-negative inhibitors of the ESCRT pathway that block HIV-1 particle production. However, Z(WT) became dependent on the presence of an L domain when NC-p1-p6 was restored to its C terminus. Furthermore, when the NC domain was replaced by a leucine zipper, the p1-p6 region, but not p6 alone, conferred sensitivity to inhibition of the ESCRT pathway. In an authentic HIV-1 Gag context, the effect of an inhibitor of the ESCRT pathway on particle production could be alleviated by deleting a portion of the NC domain together with p1. Together, these results indicate that the ESCRT pathway dependence of HIV-1 budding is determined, at least in part, by the NC-p1 region of Gag.
Figures

References
-
- Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882-11895. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

