Rearranged genomic RNA segments offer a new approach to the reverse genetics of rotaviruses
- PMID: 20427539
- PMCID: PMC2903292
- DOI: 10.1128/JVI.00547-10
Rearranged genomic RNA segments offer a new approach to the reverse genetics of rotaviruses
Abstract
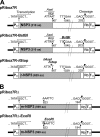
Group A rotaviruses (RV), members of the Reoviridae family, are a major cause of infantile acute gastroenteritis. The RV genome consists of 11 double-stranded RNA segments. In some cases, an RNA segment is replaced by a rearranged RNA segment, which is derived from its standard counterpart by partial sequence duplication. We report here a reverse genetics system for RV based on the preferential packaging of rearranged RNA segments. Using this system, wild-type or in vitro-engineered forms of rearranged segment 7 from a human rotavirus (encoding the NSP3 protein), derived from cloned cDNAs and transcribed in the cytoplasm of COS-7 cells with the help of T7 RNA polymerase, replaced the wild-type segment 7 of a bovine helper virus (strain RF). Recombinant RF viruses (i.e., engineered monoreassortant RF viruses) containing an exogenous rearranged RNA were recovered by propagating the viral progeny in MA-104 cells, with no need for additional selective pressure. Our findings offer the possibility to extend RV reverse genetics to segments encoding nonstructural or structural proteins for which no potent selective tools, such as neutralizing antibodies, are available. In addition, the system described here is the first to enable the introduction of a mutated gene expressing a modified nonstructural protein into an infectious RV. This reverse genetics system offers new perspectives for investigating RV protein functions and developing recombinant live RV vaccines containing specific changes targeted for attenuation.
Figures



References
-
- Aponte, C., N. M. Mattion, M. K. Estes, A. Charpilienne, and J. Cohen. 1993. Expression of two bovine rotavirus non-structural proteins (NSP2, NSP3) in the baculovirus system and production of monoclonal antibodies directed against the expressed proteins. Arch. Virol. 133:85-95. - PubMed
-
- Dehee, A., N. Schnepf, C. Deback, A. L. Poeury, and A. Garbarg-Chenon. 2006. Preferential segregation of rearranged segments in rotaviral progeny after mixed infection, abstr. PW4.1, p. 81. Abstr. 9th Int. Symp. ds-RNA Viruses.
-
- Desselberger, U. 1996. Genome rearrangements of rotaviruses. Adv. Virus Res. 46:69-95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

