Ribosome-binding proteins Mdm38 and Mba1 display overlapping functions for regulation of mitochondrial translation
- PMID: 20427570
- PMCID: PMC2883938
- DOI: 10.1091/mbc.e10-02-0101
Ribosome-binding proteins Mdm38 and Mba1 display overlapping functions for regulation of mitochondrial translation
Abstract
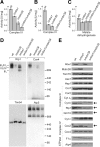
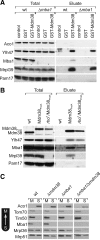
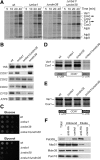
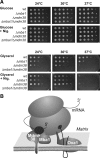
Biogenesis of respiratory chain complexes depends on the expression of mitochondrial-encoded subunits. Their synthesis occurs on membrane-associated ribosomes and is probably coupled to their membrane insertion. Defects in expression of mitochondrial translation products are among the major causes of mitochondrial disorders. Mdm38 is related to Letm1, a protein affected in Wolf-Hirschhorn syndrome patients. Like Mba1 and Oxa1, Mdm38 is an inner membrane protein that interacts with ribosomes and is involved in respiratory chain biogenesis. We find that simultaneous loss of Mba1 and Mdm38 causes severe synthetic defects in the biogenesis of cytochrome reductase and cytochrome oxidase. These defects are not due to a compromised membrane binding of ribosomes but the consequence of a mis-regulation in the synthesis of Cox1 and cytochrome b. Cox1 expression is restored by replacing Cox1-specific regulatory regions in the mRNA. We conclude, that Mdm38 and Mba1 exhibit overlapping regulatory functions in translation of selected mitochondrial mRNAs.
Figures

References
-
- Altmann K., Dürr M., Westermann B. Saccharomyces cerevisiae as a model organism to study mitochondrial biology: general considerations and basic procedures. Methods Mol. Biol. 2007;372:81–90. - PubMed
-
- Bauerschmitt H., Funes S., Herrmann J. M. The membrane-bound GTPase Guf1 promotes mitochondrial protein synthesis under suboptimal conditions. J. Biol. Chem. 2008;283:17139–17146. - PubMed
-
- Dekker P. J., Martin F., Maarse A. C., Bömer U., Müller H., Guiard B., Meijer M., Rassow J., Pfanner N. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 1997;16:5408–5419. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

