Magnetoencephalography demonstrates multiple asynchronous generators during human sleep spindles
- PMID: 20427615
- PMCID: PMC2904206
- DOI: 10.1152/jn.00198.2010
Magnetoencephalography demonstrates multiple asynchronous generators during human sleep spindles
Abstract
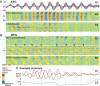
Sleep spindles are approximately 1 s bursts of 10-16 Hz activity that occur during stage 2 sleep. Spindles are highly synchronous across the cortex and thalamus in animals, and across the scalp in humans, implying correspondingly widespread and synchronized cortical generators. However, prior studies have noted occasional dissociations of the magnetoencephalogram (MEG) from the EEG during spindles, although detailed studies of this phenomenon have been lacking. We systematically compared high-density MEG and EEG recordings during naturally occurring spindles in healthy humans. As expected, EEG was highly coherent across the scalp, with consistent topography across spindles. In contrast, the simultaneously recorded MEG was not synchronous, but varied strongly in amplitude and phase across locations and spindles. Overall, average coherence between pairs of EEG sensors was approximately 0.7, whereas MEG coherence was approximately 0.3 during spindles. Whereas 2 principle components explained approximately 50% of EEG spindle variance, >15 were required for MEG. Each PCA component for MEG typically involved several widely distributed locations, which were relatively coherent with each other. These results show that, in contrast to current models based on animal experiments, multiple asynchronous neural generators are active during normal human sleep spindles and are visible to MEG. It is possible that these multiple sources may overlap sufficiently in different EEG sensors to appear synchronous. Alternatively, EEG recordings may reflect diffusely distributed synchronous generators that are less visible to MEG. An intriguing possibility is that MEG preferentially records from the focal core thalamocortical system during spindles, and EEG from the distributed matrix system.
Figures




References
-
- Achermann P, Borbely AA. Temporal evolution of coherence and power in the human sleep electroencephalogram. J Sleep Res 7Suppl. 1: 36–41, 1998 - PubMed
-
- Anderer P, Klosch G, Gruber G, Trenker E, Pascual-Marqui RD, Zeitlhofer J, Barbanoj MJ, Rappelsberger P, Saletu B. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience 103: 581–592, 2001 - PubMed
-
- Andersen P, Andersson SA. Physiological Basis of the Alpha Rhythm. New York: Meredith, 1968
-
- Buzsaki G. Rhythms of the Brain. Oxford, UK: Oxford University Press, 2006
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

