Calcium balance and mechanotransduction in rat cochlear hair cells
- PMID: 20427623
- PMCID: PMC2904212
- DOI: 10.1152/jn.00019.2010
Calcium balance and mechanotransduction in rat cochlear hair cells
Abstract
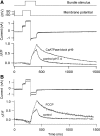
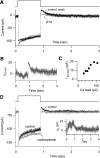
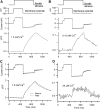
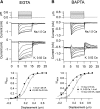
Auditory transduction occurs by opening of Ca(2+)-permeable mechanotransducer (MT) channels in hair cell stereociliary bundles. Ca(2+) clearance from bundles was followed in rat outer hair cells (OHCs) using fast imaging of fluorescent indicators. Bundle deflection caused a rapid rise in Ca(2+) that decayed after the stimulus, with a time constant of about 50 ms. The time constant was increased by blocking Ca(2+) uptake into the subcuticular plate mitochondria or by inhibiting the hair bundle plasma membrane Ca(2+) ATPase (PMCA) pump. Such manipulations raised intracellular Ca(2+) and desensitized the MT channels. Measurement of the electrogenic PMCA pump current, which saturated at 18 pA with increasing Ca(2+) loads, indicated a maximum Ca(2+) extrusion rate of 3.7 fmol x s(-1). The amplitude of the Ca(2+) transient decreased in proportion to the Ca(2+) concentration bathing the bundle and in artificial endolymph (160 mM K(+), 20 microM Ca(2+)), Ca(2+) carried 0.2% of the MT current. Nevertheless, MT currents in endolymph displayed fast adaptation with a submillisecond time constant. In endolymph, roughly 40% of the MT current was activated at rest when using 1 mM intracellular BAPTA compared with 12% with 1 mM EGTA, which enabled estimation of the in vivo Ca(2+) load as 3 pA at rest. The results were reproduced by a model of hair bundle Ca(2+) diffusion, showing that the measured PMCA pump density could handle Ca(2+) loads incurred from resting and maximal MT currents in endolymph. The model also indicated the endogenous mobile buffer was equivalent to 1 mM BAPTA.
Figures





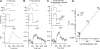

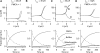
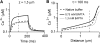
References
-
- Apicella S, Chen S, Bing R, Penniston JT, Llinás R, Hillman DE. Plasmalemmal ATPase calcium pump localizes to inner and outer hair bundles. Neuroscience 79: 1145–1151, 1997 - PubMed
-
- Babcock DF, Hille B. Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol 8: 398–404, 1998 - PubMed
-
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58: 495–505, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

