Phase 2 and pharmacodynamic study of oral forodesine in patients with advanced, fludarabine-treated chronic lymphocytic leukemia
- PMID: 20427701
- PMCID: PMC2924226
- DOI: 10.1182/blood-2010-02-272039
Phase 2 and pharmacodynamic study of oral forodesine in patients with advanced, fludarabine-treated chronic lymphocytic leukemia
Abstract
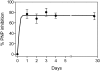
Forodesine is a new and potent purine nucleoside phosphorylase (PNP) inhibitor. Patients with chronic lymphocytic leukemia (CLL) with primary resistance to fludarabine-based therapy or with progressive disease were eligible for oral forodesine (200 mg/d) for up to 24 weeks. Eight patients with median lymphocyte count of 35.9 x 10(9)/L and median serum beta2 microglobulin level of 6.45 mg/L were treated. Six had Rai stage III to IV and were previously heavily treated (median prior therapy = 5). Two had transient decrease in lymphocyte count to normal, whereas in 5, disease progressed. Adverse events were mild. Steady-state level of forodesine ranged from 200 to 1300 nM and did not reach desired 2 microM level. PNP inhibition ranged from 57% to 89% and steady-state 2'-deoxyguanosine (dGuo) concentration median was 1.8 microM. Intracellular deoxyguanosine triphosphate (dGTP) increase was very modest, from median of 6 microM to 10 microM. Compared with in vivo, in vitro incubations of CLL lymphocytes with 10 or 20 microM dGuo and forodesine (2 microM) resulted in accumulation of higher levels of dGTP (40-250 microM) which resulted in increase in apoptosis. Forodesine has biologic activity in CLL; pharmacodynamic parameters suggest that an alternate dosing schedule and/or higher doses to achieve greater intracellular dGTP may be beneficial in this patient population.
Trial registration: ClinicalTrials.gov NCT00289549.
Figures



 ) or forodesine and dGuo–treated (
) or forodesine and dGuo–treated ( ) cells (A). Data from Figure 4A are plotted with data from Figure 5A to compare in vivo (
) cells (A). Data from Figure 4A are plotted with data from Figure 5A to compare in vivo ( ) and in vitro (
) and in vitro ( ) accumulation of dGTP (B). In the same cells, cell death was analyzed at 24 hours by Annexin positivity procedure as described in “Measurement of cell viability” in untreated (
) accumulation of dGTP (B). In the same cells, cell death was analyzed at 24 hours by Annexin positivity procedure as described in “Measurement of cell viability” in untreated ( ) and forodesine and dGuo–treated (
) and forodesine and dGuo–treated ( ) cells (C).
) cells (C).References
-
- Keating MJ, O'Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43(9):1755–1762. - PubMed
-
- Parks RJ, Agarwal RP. In: The Enzymes. Boyer PD, editor. New York, NY: Academic Press; 1972. pp. 483–514.
-
- Voet D, Voet JG, Pratt CW, editors. Fundamentals of Biochemistry: Life at the Molecular Level. New York, NY: John Wiley & Sons Inc; pp. 817–847.
-
- Scharenberg JG, Spaapen LJ, Rijkers GT, Wadman SK, Staal GE, Zegers BJ. Mechanisms of deoxyguanosine toxicity for human T and B lymphocytes. Adv Exp Med Biol. 1986;195(Pt B):191–199. - PubMed
-
- Giblett ER, Ammann AJ, Wara DW, Sandman R, Diamond LK. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975;1(7914):1010–1013. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

