Recent advances in noble metal nanocatalysts for Suzuki and Heck cross-coupling reactions
- PMID: 20428032
- PMCID: PMC6257342
- DOI: 10.3390/molecules15042124
Recent advances in noble metal nanocatalysts for Suzuki and Heck cross-coupling reactions
Abstract
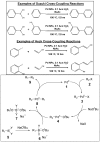
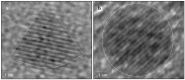
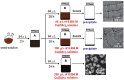
Since metal nanoparticles have a high surface-to-volume ratio and very active surface atoms, they are very attractive catalysts for a wide variety of organic and inorganic reactions, compared to bulk catalysts. Metal nanoparticles suspended in colloidal solutions and those adsorbed onto bulk supports have been used as catalysts for a wide variety of carbon-carbon bond formation reactions such as the Suzuki and Heck cross-coupling reactions. This review article highlights some of the latest advances in the application of noble metal nanoparticles as catalysts for these two industrially important classes of cross-coupling reactions. We will discuss several important advances in using metal nanocatalysts in Suzuki and Heck cross-coupling reactions such as investigations on the nanoparticle shape dependence on the catalytic activity, novel types of supported metal nanoparticles as nanocatalysts, and the use of bi-metallic, tri-metallic and multi-metallic nanoparticles as catalysts for the Suzuki and Heck cross-coupling reactions.
Figures




Similar articles
-
"Homeopathic" palladium nanoparticle catalysis of cross carbon-carbon coupling reactions.Acc Chem Res. 2014 Feb 18;47(2):494-503. doi: 10.1021/ar400168s. Epub 2013 Nov 11. Acc Chem Res. 2014. PMID: 24215156
-
Recent Progress in Application of Graphene Supported Metal Nanoparticles in C-C and C-X Coupling Reactions.Chem Rec. 2018 Feb;18(2):165-229. doi: 10.1002/tcr.201700022. Epub 2017 Jul 26. Chem Rec. 2018. PMID: 28745452 Review.
-
Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability.J Phys Chem B. 2005 Jul 7;109(26):12663-76. doi: 10.1021/jp051066p. J Phys Chem B. 2005. PMID: 16852568
-
Intracellular Catalysis with Selected Metal Complexes and Metallic Nanoparticles: Advances toward the Development of Catalytic Metallodrugs.Chem Rev. 2019 Jan 23;119(2):829-869. doi: 10.1021/acs.chemrev.8b00493. Epub 2019 Jan 8. Chem Rev. 2019. PMID: 30618246 Review.
-
The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions.Molecules. 2018 Jul 10;23(7):1676. doi: 10.3390/molecules23071676. Molecules. 2018. PMID: 29996491 Free PMC article. Review.
Cited by
-
Comparative Study of the Frech Catalyst with Two Conventional Catalysts in the Heck Synthesis of 2,4-Diaminopyrimidine-based Antibiotics.Org Prep Proced Int. 2013;45(1):66-71. doi: 10.1080/00304948.2013.743755. Org Prep Proced Int. 2013. PMID: 23788820 Free PMC article. No abstract available.
-
Fullerene fine particles adhere to pollen grains and affect their autofluorescence and germination.Nanotechnol Sci Appl. 2011 May 20;4:67-71. doi: 10.2147/NSA.S14263. eCollection 2011. Nanotechnol Sci Appl. 2011. PMID: 24198486 Free PMC article.
-
Recyclable LaF3·Pd nanocatalyst in Suzuki coupling: green synthesis of biaryls from haloarenes and phenylboronic acids.RSC Adv. 2024 Jul 5;14(30):21269-21276. doi: 10.1039/d4ra00686k. eCollection 2024 Jul 5. RSC Adv. 2024. PMID: 38974224 Free PMC article.
-
2,5-Diisopropenylthiophene by Suzuki-Miyaura cross-coupling reaction and its exploitation in inverse vulcanization: a case study.RSC Adv. 2022 Mar 22;12(15):8924-8935. doi: 10.1039/d2ra00654e. eCollection 2022 Mar 21. RSC Adv. 2022. PMID: 35424896 Free PMC article.
-
Prospects and Applications of Palladium Nanoparticles in the Cross-coupling of (hetero)aryl Halides and Related Analogues.ChemistryOpen. 2021 Apr;10(4):430-450. doi: 10.1002/open.202000309. Epub 2021 Feb 15. ChemistryOpen. 2021. PMID: 33590728 Free PMC article. Review.
References
-
- Miyaura N., Yanagi T., Suzuki A. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synth. Commun. 1981;11:513–519. doi: 10.1080/00397918108063618. - DOI
-
- Suzuki A. Synthetic Studies via the Cross-Coupling Reaction of Organoboron Derivatives with Organic Halides. Pure Appl. Chem. 1991;63:419–422. doi: 10.1351/pac199163030419. - DOI
-
- Miyaura N., Suzuki A. A Stereospecific Synthesis of Conjugated (E,Z)- and (Z,Z)-Alkadienes by a Palladium-Catalyzed Cross-Coupling Reaction of 1-Alkenylboranes with 1-Alkenyl Bromides. Tetrahedron Lett. 1981;22:127–130. doi: 10.1016/0040-4039(81)80166-9. - DOI
-
- Miyaura N., Yamada K., Suzuki A. A New Stereospecific Cross-Coupling by the Palladium-Catalyzed Reaction of 1-Alkenylboranes With 1-Alkenyl or 1-Alkynyl Halides. Tetrahedron Lett. 1979;20:3437–3440. doi: 10.1016/S0040-4039(01)95429-2. - DOI
-
- Miyaura N., Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

