rAAV2/5 gene-targeting to rods:dose-dependent efficiency and complications associated with different promoters
- PMID: 20428215
- PMCID: PMC2914811
- DOI: 10.1038/gt.2010.56
rAAV2/5 gene-targeting to rods:dose-dependent efficiency and complications associated with different promoters
Abstract
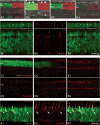
A prerequisite for using corrective gene therapy to treat humans with inherited retinal degenerative diseases that primarily affect rods is to develop viral vectors that target specifically this population of photoreceptors. The delivery of a viral vector with photoreceptor tropism coupled with a rod-specific promoter is likely to be the safest and most efficient approach to target expression of the therapeutic gene to rods. Three promoters that included a fragment of the proximal mouse opsin promoter (mOP), the human G-protein-coupled receptor protein kinase 1 promoter (hGRK1), or the cytomegalovirus immediate early enhancer combined with the chicken β actin proximal promoter CBA were evaluated for their specificity and robustness in driving GFP reporter gene expression in rods, when packaged in a recombinant adeno-associated viral vector of serotype 2/5 (AAV2/5), and delivered via subretinal injection to the normal canine retina. Photoreceptor-specific promoters (mOP, hGRK1) targeted robust GFP expression to rods, whereas the ubiquitously expressed CBA promoter led to transgene expression in the retinal pigment epithelium, rods, cones and rare Müller, horizontal and ganglion cells. Late onset inflammation was frequently observed both clinically and histologically with all three constructs when the highest viral titers were injected. Cone loss in the injected regions of the retinas that received the highest titers occurred with both the hGRK1 and CBA promoters. Efficient and specific rod transduction, together with preservation of retinal structure was achieved with both mOP and hGRK1 promoters when viral titers in the order of 10(11)vg ml(-1) were used.
Conflict of interest statement
Figures





References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. - PubMed
-
- Pang JJ, Chang B, Kumar A, Nusinowitz S, Noorwez SM, Li J, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13(3):565–572. - PubMed
-
- Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7(6):774–781. - PubMed
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

