Enantioselective protonation
- PMID: 20428461
- PMCID: PMC2860147
- DOI: 10.1038/nchem.297
Enantioselective protonation
Abstract
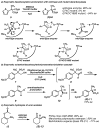
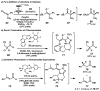
Enantioselective protonation is a common process in biosynthetic sequences. The decarboxylase and esterase enzymes that effect this valuable transformation are able to control both the steric environment around the proton acceptor (typically an enolate) and the proton donor (typically a thiol). Recently, several chemical methods to achieve enantioselective protonation have been developed by exploiting various means of enantiocontrol in different mechanisms. These laboratory transformations have proven useful for the preparation of a number of valuable organic compounds.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
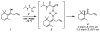





References
-
- Fehr C. Enantioselective protonation of enolates and enols. Angew Chem, Int Ed Engl. 1996;35:2566–2587.
-
- Yanagisawa A, Ishihara K, Yamamoto H. Asymmetric protonations of enol derivatives. Synlett. 1997:411–420.
-
- Eames J, Weerasooriya N. Recent advances into the enantioselective protonation of protostereogenic enol derivatives. Tetrahedron: Asymmetry. 2001;12:1–24.
-
- Duhamel L, Duhamel P, Plaquevent JC. Enantioselective protonations: Fundamental insights and new concepts. Tetrahedron: Asymmetry. 2004;15:3653–3691.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

