Cortical hubs form a module for multisensory integration on top of the hierarchy of cortical networks
- PMID: 20428515
- PMCID: PMC2859882
- DOI: 10.3389/neuro.11.001.2010
Cortical hubs form a module for multisensory integration on top of the hierarchy of cortical networks
Abstract
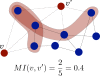
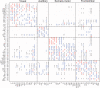
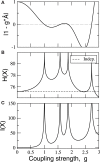
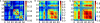
Sensory stimuli entering the nervous system follow particular paths of processing, typically separated (segregated) from the paths of other modal information. However, sensory perception, awareness and cognition emerge from the combination of information (integration). The corticocortical networks of cats and macaque monkeys display three prominent characteristics: (i) modular organisation (facilitating the segregation), (ii) abundant alternative processing paths and (iii) the presence of highly connected hubs. Here, we study in detail the organisation and potential function of the cortical hubs by graph analysis and information theoretical methods. We find that the cortical hubs form a spatially delocalised, but topologically central module with the capacity to integrate multisensory information in a collaborative manner. With this, we resolve the underlying anatomical substrate that supports the simultaneous capacity of the cortex to segregate and to integrate multisensory information.
Keywords: cortical hubs; corticocortical networks; integration; multisensory integration; segregation.
Figures

References
-
- Anthonisse J. M. (1971). The rush in a directed graph. Technical Report BN9/71. Amsterdam, Stichting Mathematisch Centrum
-
- Bear M. F., Connors B. W., Paradiso M. A. (2006). Neuroscience: Exploring the Brain. Baltimore, MD, Lippincott Williams and Wilkins
-
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 1 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

