Conformational gating of electron transfer from the nitrogenase Fe protein to MoFe protein
- PMID: 20429505
- PMCID: PMC2892898
- DOI: 10.1021/ja101737f
Conformational gating of electron transfer from the nitrogenase Fe protein to MoFe protein
Abstract
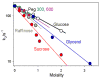
The nitrogenase Fe protein contains a [4Fe-4S] cluster and delivers one electron at a time to the catalytic MoFe protein. During this electron delivery, the Fe protein in its [4Fe-4S](1+) reduced state (Fe(red)) binds two MgATP and forms a complex with the MoFe protein, with subsequent transfer of one electron to the MoFe protein in a reaction coupled to the hydrolysis of two ATP. Crystal structures with the nitrogenase complex in different nucleotide-bound states show major conformational changes which provide a structural underpinning to suggestions that intercomponent electron transfer (ET) is "gated" by conformational changes of the complex and/or of its component proteins. Although electron delivery is coupled to ATP hydrolysis, their connection is puzzling, for it appears that ET precedes both ATP hydrolysis and Pi release. We here test the gating hypothesis with studies of the intracomplex oxidation of Fe(red) by MoFe protein in the presence of a variety of solutes. Conformational control of this process (gating) is revealed by the finding that it responds to changes in osmotic pressure (but not viscosity), with no fewer than 80 waters being bound during the reaction. The absence of a solvent kinetic isotope effect further implies that ATP hydrolysis does not occur during the rate-limiting step of ET.
Figures


References
-
- Burgess BK, Lowe DJ. Chemical Reviews (Washington, D C) 1996;96:2983–3011. - PubMed
-
- Tezcan FA, Kaiser JT, Mustafi D, Walton MY, Howard JB, Rees DC. Science (Washington, DC, U S) 2005;309:1377–1380. - PubMed
-
- Rees DC, Tezcan FA, Haynes CA, Walton MY, Andrade S, Einsle O, Howard JB. Phil Trans R Soc A. 2005;363:971–984. - PubMed
-
- Lanzilotta WN, Parker VD, Seefeldt LC. Biochemistry. 1998;37:399–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

