Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey
- PMID: 20430079
- PMCID: PMC2941552
- DOI: 10.1016/j.heares.2010.03.084
Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey
Abstract
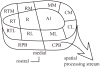
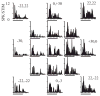
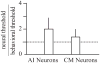
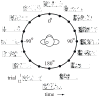
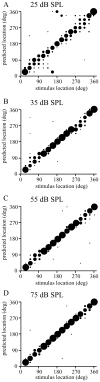
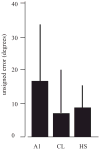
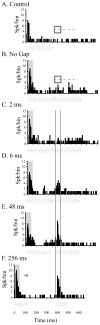
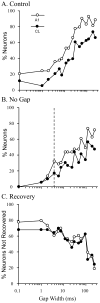
The auditory cortex is known to be a necessary neural structure for the perception of acoustic signals, particularly the spatial location and the temporal features of complex auditory stimuli. Previous studies have indicated that there is no topographic map of acoustic space in the auditory cortex and it has been proposed that spatial locations are represented by some sort of population code. Additionally, in spite of temporal processing deficits being one of the hallmark consequences of normal aging, the temporal coding of acoustic stimuli remains poorly understood. This report will address these two issues by discussing the results from several studies describing responses of single auditory cortical neurons in the non-human primate. First, we will review studies that have addressed potential spike-rate population codes of acoustic space in the caudal belt of auditory cortex. Second, we will present new data on the neuronal responses to gap stimuli in aged monkeys and compare them to published reports of gap detection thresholds. Together these studies indicate that the alert macaque monkey is an excellent model system to study both spatial and temporal processing in the auditory cortex at the single neuron level.
© 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Effects of aging on the response of single neurons to amplitude-modulated noise in primary auditory cortex of rhesus macaque.J Neurophysiol. 2016 Jun 1;115(6):2911-23. doi: 10.1152/jn.01098.2015. Epub 2016 Mar 2. J Neurophysiol. 2016. PMID: 26936987 Free PMC article.
-
Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey.J Neurophysiol. 2006 Dec;96(6):3323-37. doi: 10.1152/jn.00392.2006. Epub 2006 Aug 30. J Neurophysiol. 2006. PMID: 16943318
-
Functional specialization in rhesus monkey auditory cortex.Science. 2001 Apr 13;292(5515):290-3. doi: 10.1126/science.1058911. Science. 2001. PMID: 11303104
-
Distributed coding of sound locations in the auditory cortex.Biol Cybern. 2003 Nov;89(5):341-9. doi: 10.1007/s00422-003-0439-1. Epub 2003 Nov 12. Biol Cybern. 2003. PMID: 14669014 Review.
-
Processing of complex sounds in the auditory cortex of cat, monkey, and man.Acta Otolaryngol Suppl. 1997;532:34-8. doi: 10.3109/00016489709126142. Acta Otolaryngol Suppl. 1997. PMID: 9442842 Review.
Cited by
-
Characterizing cognitive aging of associative memory in animal models.Front Aging Neurosci. 2012 Sep 12;4:10. doi: 10.3389/fnagi.2012.00010. eCollection 2012. Front Aging Neurosci. 2012. PMID: 22988435 Free PMC article.
-
Effects of aging on the response of single neurons to amplitude-modulated noise in primary auditory cortex of rhesus macaque.J Neurophysiol. 2016 Jun 1;115(6):2911-23. doi: 10.1152/jn.01098.2015. Epub 2016 Mar 2. J Neurophysiol. 2016. PMID: 26936987 Free PMC article.
-
Reversal of age-related neural timing delays with training.Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):4357-62. doi: 10.1073/pnas.1213555110. Epub 2013 Feb 11. Proc Natl Acad Sci U S A. 2013. PMID: 23401541 Free PMC article. Clinical Trial.
-
Age-related changes in auditory nerve-inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice.Neuroscience. 2015 Apr 30;292:22-33. doi: 10.1016/j.neuroscience.2015.01.068. Epub 2015 Feb 7. Neuroscience. 2015. PMID: 25665752 Free PMC article.
-
A dynamic auditory-cognitive system supports speech-in-noise perception in older adults.Hear Res. 2013 Jun;300:18-32. doi: 10.1016/j.heares.2013.03.006. Epub 2013 Mar 27. Hear Res. 2013. PMID: 23541911 Free PMC article.
References
-
- Altshuler MW, Comalli PE. Effect of stimulus intensity and frequency on median horizontal plane sound localization. J Auditory Res. 1975;15:262–265.
-
- Beitel RE, Kaas JH. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol. 1993;70:351–369. - PubMed
-
- Bennett CL, Davis RT, Miller JM. Demonstration of presbycusis across repeated measures in a nonhuman primate species. Behav Neurosci. 1983;97:602–607. - PubMed
-
- Blauert J. The Psychophysics of Human Sound Localization. 2. MIT Press; 1997. Spatial Hearing.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

