Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32
- PMID: 20430082
- PMCID: PMC8204221
- DOI: 10.1016/j.heares.2010.04.003
Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32
Abstract
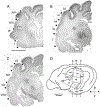
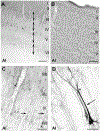
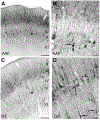
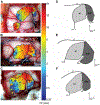
The monoclonal antibody SMI-32 was used to characterize and distinguish individual areas of cat auditory cortex. SMI-32 labels non-phosphorylated epitopes on the high- and medium-molecular weight subunits of neurofilament proteins in cortical pyramidal cells and dendritic trees with the most robust immunoreactivity in layers III and V. Auditory areas with unique patterns of immunoreactivity included: primary auditory cortex (AI), second auditory cortex (AII), dorsal zone (DZ), posterior auditory field (PAF), ventral posterior auditory field (VPAF), ventral auditory field (VAF), temporal cortex (T), insular cortex (IN), anterior auditory field (AAF), and the auditory field of the anterior ectosylvian sulcus (fAES). Unique patterns of labeling intensity, soma shape, soma size, layers of immunoreactivity, laminar distribution of dendritic arbors, and labeled cell density were identified. Features that were consistent in all areas included: layers I and IV neurons are immunonegative; nearly all immunoreactive cells are pyramidal; and immunoreactive neurons are always present in layer V. To quantify the results, the numbers of labeled cells and dendrites, as well as cell diameter, were collected and used as tools for identifying and differentiating areas. Quantification of the labeling patterns also established profiles for ten auditory areas/layers and their degree of immunoreactivity. Areal borders delineated by SMI-32 were highly correlated with tonotopically-defined areal boundaries. Overall, SMI-32 immunoreactivity can delineate ten areas of cat auditory cortex and demarcate topographic borders. The ability to distinguish auditory areas with SMI-32 is valuable for the identification of auditory cerebral areas in electrophysiological, anatomical, and/or behavioral investigations.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures













Similar articles
-
Neurofilament protein: a selective marker for the architectonic parcellation of the visual cortex in adult cat brain.J Comp Neurol. 2001 Dec 24;441(4):345-68. doi: 10.1002/cne.1416. J Comp Neurol. 2001. PMID: 11745654
-
Regional analysis of neurofilament protein immunoreactivity in the hamster's cortex.J Chem Neuroanat. 2005 May;29(3):193-208. doi: 10.1016/j.jchemneu.2005.01.003. J Chem Neuroanat. 2005. PMID: 15820621
-
Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas.Exp Brain Res. 1991;86(3):483-505. doi: 10.1007/BF00230523. Exp Brain Res. 1991. PMID: 1722171
-
Processing of complex sounds in the auditory cortex of cat, monkey, and man.Acta Otolaryngol Suppl. 1997;532:34-8. doi: 10.3109/00016489709126142. Acta Otolaryngol Suppl. 1997. PMID: 9442842 Review.
-
Area 4 has layer IV in adult primates.Eur J Neurosci. 2014 Jun;39(11):1824-34. doi: 10.1111/ejn.12585. Epub 2014 Apr 16. Eur J Neurosci. 2014. PMID: 24735460 Free PMC article. Review.
Cited by
-
A comparison of multisensory features of two auditory cortical areas: primary (A1) and higher-order dorsal zone (DZ).Cereb Cortex Commun. 2022 Nov 17;4(1):tgac049. doi: 10.1093/texcom/tgac049. eCollection 2023. Cereb Cortex Commun. 2022. PMID: 36632047 Free PMC article.
-
Behavioral modulation of neural encoding of click-trains in the primary and nonprimary auditory cortex of cats.J Neurosci. 2013 Aug 7;33(32):13126-37. doi: 10.1523/JNEUROSCI.1724-13.2013. J Neurosci. 2013. PMID: 23926266 Free PMC article.
-
Frequency transformation in the auditory lemniscal thalamocortical system.Front Neural Circuits. 2014 Jul 8;8:75. doi: 10.3389/fncir.2014.00075. eCollection 2014. Front Neural Circuits. 2014. PMID: 25071456 Free PMC article. Review.
-
Intrinsic Connections of the Core Auditory Cortical Regions and Rostral Supratemporal Plane in the Macaque Monkey.Cereb Cortex. 2017 Jan 1;27(1):809-840. doi: 10.1093/cercor/bhv277. Cereb Cortex. 2017. PMID: 26620266 Free PMC article.
-
Contributions of Parietal Cortex to the Working Memory of an Obstacle Acquired Visually or Tactilely in the Locomoting Cat.Cereb Cortex. 2018 Sep 1;28(9):3143-3158. doi: 10.1093/cercor/bhx186. Cereb Cortex. 2018. PMID: 28981640 Free PMC article.
References
-
- Ashwell KWS, Zhang L-L, Marotte LR, 2005. Cyto-and chemoarchitecture of the cortex of the tammar wallaby (macropus eugenii): areal organization. Brain Behav. Evol 66, 114–136. - PubMed
-
- Baldauf ZB, 2005. SMI-32 parcellates the visual cortical areas of the marmoset. Neurosci. Lett 383, 1–2. - PubMed
-
- Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, Bennett BT, Pascoe P, Schull E, Cork LC, Francis-Floyd R, Amass KD, Johnson RJ, Schmidt RH, Underwood W, Thorton GW, Kohn B, 2001. 2000 Report of the American Veterinary Medical Association Panel of Euthanasia. J. Am. Vet. Med. Assoc 218, 669–696. - PubMed
-
- Beneyto M, Winer JA, Larue DT, Prieto JJ, 1998. Auditory connections and neurochemistry of the sagulum. J. Comp. Neurol 401, 329–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

