New insights in the immunologic basis of psoriasis
- PMID: 20430301
- PMCID: PMC2868373
- DOI: 10.1016/j.sder.2010.03.001
New insights in the immunologic basis of psoriasis
Abstract
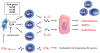
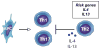
Psoriasis vulgaris is a multifactorial heritable disease characterized by severe inflammation resulting in poorly differentiated, hyperproliferative keratinocytes. Recent advances in genetic analyses have implicated components regulating the interleukin (IL)-23 and nuclear factor-kappaB pathways as risk factors for psoriasis. These inflammatory pathways exhibit increased activity in skin lesions, and promote secretion of various cytokines, such as IL-17 and IL-22. Unrestrained, the activated inflammatory cytokine network in psoriasis may trigger a vicious cycle of inflammation and cellular proliferation that ultimately results in lesion formation. These advances in genetic analyses, together with the progress made in targeted biological therapy, pave the path to tailor treatment on the basis of an individual's genetic and immunologic profile.
Conflict of interest statement
Disclosure: No relevant conflicts of interest in relations to this article.
Figures



References
-
- Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. - PubMed
-
- Rongioletti F, Fiorucci C, Parodi A. Psoriasis induced or aggravated by drugs. J Rheumatol Suppl. 2009;83:59–61. - PubMed
-
- Gudjonsson J, Elder J. Psoriasis. In: Wolff K, Goldsmith L, Katz S, et al., editors. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill; 2007.
-
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. - PubMed
-
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256(22):3110–3116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
