The effects of intraspinal microstimulation on spinal cord tissue in the rat
- PMID: 20430436
- PMCID: PMC2875271
- DOI: 10.1016/j.biomaterials.2010.03.051
The effects of intraspinal microstimulation on spinal cord tissue in the rat
Abstract
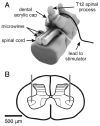
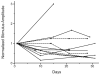
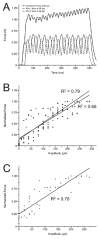
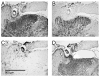
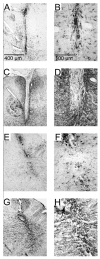
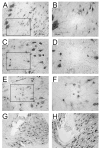
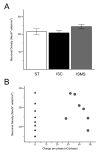
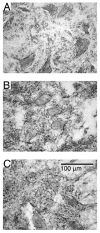
Intraspinal microstimulation (ISMS) involves the implantation of microwires into the spinal cord below the level of an injury to excite neural networks involved in the control of locomotion in the lower limbs. The goal of this study was to examine the potential spinal cord damage that might occur with chronic ISMS. We employed functional measures of force recruitment and immunohistochemical processing of serial spinal cord sections to evaluate any damage induced by spinal transection, implantation of ISMS arrays, and electrical stimulation of 4h/day for 30 days. Functional measurements showed no change in force recruitment following transection and chronic ISMS, indicating no changes to underlying neural networks. The implantation of sham intraspinal microwires produced a spatially-limited increase in the density of microglia/macrophages and GFAP+ astrocytes adjacent to the microwire tracks, indicating a persistent immune response. Most importantly, these results were not different from those around microwires that were chronically pulsed with charge levels up to 48nC/phase. Likewise, measurements of neuronal density indicated no decrease in neuronal cell bodies in the ventral grey matter surrounding ISMS microwires (243.6/mm2+/-35.3/mm2) compared to tissue surrounding sham microwires (207.8/mm2+/-38.8/mm2). We conclude that the implantation of intraspinal microwires and chronic application of ISMS are well tolerated by spinal cord tissue.
Crown Copyright (c) 2010. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Popovic DB. Neural Prostheses for Movement Restoration. In: Moore J, Zouridakis G, editors. Biomedical Technology and Devices Handbook. CRC Press LLC; 2004. pp. 28–1.
-
- Mushahwar VK, Jacobs PL, Normann RA, Triolo RJ, Kleitman N. New functional electrical stimulation approaches to standing and walking. J Neural Eng. 2007;4:S181–97. - PubMed
-
- Lau B, Guevremont L, Mushahwar VK. Strategies for generating prolonged functional standing using intramuscular stimulation or intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2007;15:273–85. - PubMed
-
- Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:430–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

