Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract
- PMID: 20430747
- PMCID: PMC2860252
- DOI: 10.1242/dev.044586
Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract
Abstract
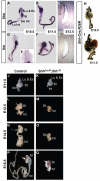
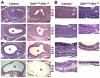
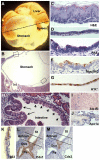
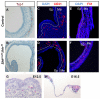
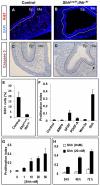
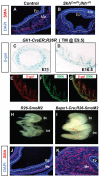
Homeostasis of the vertebrate digestive tract requires interactions between an endodermal epithelium and mesenchymal cells derived from the splanchnic mesoderm. Signaling between these two tissue layers is also crucial for patterning and growth of the developing gut. From early developmental stages, sonic hedgehog (Shh) and indian hedgehog (Ihh) are secreted by the endoderm of the mammalian gut, indicative of a developmental role. Further, misregulated hedgehog (Hh) signaling is implicated in both congenital defects and cancers arising from the gastrointestinal tract. In the mouse, only limited gastrointestinal anomalies arise following removal of either Shh or Ihh. However, given the considerable overlap in their endodermal expression domains, a functional redundancy between these signals might mask a more extensive role for Hh signaling in development of the mammalian gut. To address this possibility, we adopted a conditional approach to remove both Shh and Ihh functions from early mouse gut endoderm. Analysis of compound mutants indicates that continuous Hh signaling is dispensable for regional patterning of the gut tube, but is essential for growth of the underlying mesenchyme. Additional in vitro analysis, together with genetic gain-of-function studies, further demonstrate that Hh proteins act as paracrine mitogens to promote the expansion of adjacent mesenchymal progenitors, including those of the smooth muscle compartment. Together, these studies provide new insights into tissue interactions underlying mammalian gastrointestinal organogenesis and disease.
Figures
References
-
- Ahn S., Joyner A. L. (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505-516 - PubMed
-
- Apelqvist A., Ahlgren U., Edlund H. (1997). Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 7, 801-804 - PubMed
-
- Arsic D., Qi B. Q., Beasley S. W. (2002). Hedgehog in the human: a possible explanation for the VATER association. J. Paediatr. Child Health 38, 117-121 - PubMed
-
- Aubin J., Dery U., Lemieux M., Chailler P., Jeannotte L. (2002). Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129, 4075-4087 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

