Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen
- PMID: 20430889
- PMCID: PMC2898360
- DOI: 10.1074/jbc.M110.121319
Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen
Abstract
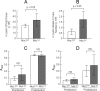
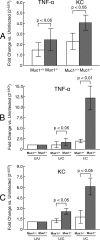
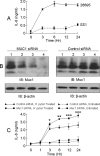
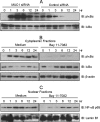
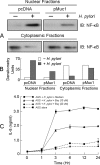
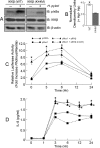
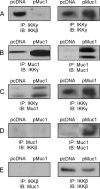
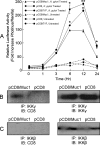
Helicobacter pylori infection of the gastric mucosa causes an active-chronic inflammation that is strongly linked to the development of duodenal and gastric ulcers and stomach cancer. However, greater than 80% of individuals infected with H. pylori are asymptomatic beyond histologic inflammation, and it is unknown what factors influence the incidence and character of bacterial-associated gastritis and related disorders. Because previous studies demonstrated that the Muc1 epithelial glycoprotein inhibited inflammation during acute lung infection by Pseudomonas aeruginosa, we asked whether Muc1 might also counter-regulate gastric inflammation in response to H. pylori infection. Muc1(-/-) mice displayed increased bacterial colonization of the stomach and greater TNF-alpha and keratinocyte chemoattractant transcript levels compared with Muc1(+/+) mice after experimental H. pylori infection. Knockdown of Muc1 expression in AGS human gastric epithelial cells by RNA interference was associated with increased phosphorylation of IkappaBalpha, augmented activation and nuclear translocation of NF-kappaB, and enhanced production of interleulin-8 compared with Muc1-expressing cells. Conversely, Muc1 overexpression was correlated with decreased NF-kappaB activation, reduced interleulin-8 production, and diminished IkappaB kinase beta (IKKbeta)/IKKgamma coimmunoprecipitation compared with cells expressing Muc1 endogenously. Cotransfection of AGS cells with Muc1 plus IKKbeta, but not a catalytically inactive IKKbeta mutant, reversed the Muc1 inhibitory effect. Finally, Muc1 formed a coimmunoprecipitation complex with IKKgamma but not with IKKbeta. These results are consistent with the hypothesis that Muc1 binds to IKKgamma, thereby inhibiting formation of the catalytically active IKK complex and blocking the ability of H. pylori to stimulate IkappaBalpha phosphorylation, NF-kappaB activation, and downstream inflammatory responses.
Figures


References
-
- Levine S. J. (1995) J. Investig. Med. 43, 241–249 - PubMed
-
- Lu W., Hisatsune A., Koga T., Kato K., Kuwahara I., Lillehoj E. P., Chen W., Cross A. S., Gendler S. J., Gewirtz A. T., Kim K. C. (2006) J. Immunol. 176, 3890–3894 - PubMed
-
- Kardon R., Price R. E., Julian J., Lagow E., Tseng S. C., Gendler S. J., Carson D. D. (1999) Invest. Ophthalmol. Vis. Sci. 40, 1328–1335 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

