Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease
- PMID: 20431041
- PMCID: PMC2900957
- DOI: 10.1681/ASN.2009121291
Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease
Abstract
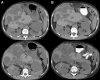
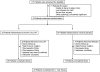
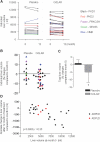
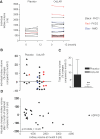
There are no proven, effective therapies for polycystic kidney disease (PKD) or polycystic liver disease (PLD). We enrolled 42 patients with severe PLD resulting from autosomal dominant PKD (ADPKD) or autosomal dominant PLD (ADPLD) in a randomized, double-blind, placebo-controlled trial of octreotide, a long-acting somatostatin analogue. We randomly assigned 42 patients in a 2:1 ratio to octreotide LAR depot (up to 40 mg every 28+/-5 days) or placebo for 1 year. The primary end point was percent change in liver volume from baseline to 1 year, measured by MRI. Secondary end points were changes in total kidney volume, GFR, quality of life, safety, vital signs, and clinical laboratory tests. Thirty-four patients had ADPKD, and eight had ADPLD. Liver volume decreased by 4.95%+/-6.77% in the octreotide group but remained practically unchanged (+0.92%+/-8.33%) in the placebo group (P=0.048). Among patients with ADPKD, total kidney volume remained practically unchanged (+0.25%+/-7.53%) in the octreotide group but increased by 8.61%+/-10.07% in the placebo group (P=0.045). Changes in GFR were similar in both groups. Octreotide was well tolerated; treated individuals reported an improved perception of bodily pain and physical activity. In summary, octreotide slowed the progressive increase in liver volume and total kidney volume, improved health perception among patients with PLD, and had an acceptable side effect profile.
Figures
Comment in
-
Randomized intervention studies in human polycystic kidney and liver disease.J Am Soc Nephrol. 2010 Jun;21(6):891-3. doi: 10.1681/ASN.2010030262. Epub 2010 Apr 29. J Am Soc Nephrol. 2010. PMID: 20431043 No abstract available.
-
Polycystic kidney disease: Promising new potential therapies for patients with autosomal dominant polycystic kidney disease.Nat Rev Nephrol. 2010 Aug;6(8):443. doi: 10.1038/nrneph.2010.87. Nat Rev Nephrol. 2010. PMID: 20690198 No abstract available.
References
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007. - PubMed
-
- Davila S, Furu L, Gharavi AG, Tian X, Onoe T, Qian Q, Li A, Cai Y, Kamath PS, King BF, Azurmendi PJ, Tahvanainen P, Kaariainen H, Hockerstedt K, Devuyst O, Pirson Y, Martin RS, Lifton RP, Tahvanainen E, Torres VE, Somlo S: Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet 36: 575–577, 2004. - PubMed
-
- Drenth JP, te Morsche RH, Smink R, Bonifacino JS, Jansen JB: Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat Genet 33: 345–347, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

