Stroke team remote evaluation using a digital observation camera in Arizona: the initial mayo clinic experience trial
- PMID: 20431081
- PMCID: PMC2876204
- DOI: 10.1161/STROKEAHA.109.574509
Stroke team remote evaluation using a digital observation camera in Arizona: the initial mayo clinic experience trial
Abstract
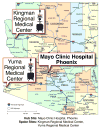
Background and purpose: Telemedicine techniques can be used to address the rural-metropolitan disparity in acute stroke care. The Stroke Team Remote Evaluation Using a Digital Observation Camera (STRokE DOC) trial reported more accurate decision making for telemedicine consultations compared with telephone-only and that the California-based research network facilitated a high rate of thrombolysis use, improved data collection, low risk of complications, low technical complications, and favorable assessment times. The main objective of the STRokE DOC Arizona TIME (The Initial Mayo Clinic Experience) trial was to determine the feasibility of establishing, de novo, a single-hub, multirural spoke hospital telestroke research network across a large geographical area in Arizona by replicating the STRokE DOC protocol.
Methods: Methods included prospective, single-hub, 2-spoke, randomized, blinded, controlled trial of a 2-way, site-independent, audiovisual telemedicine system designed for remote examination of adult patients with acute stroke versus telephone consultation to assess eligibility for treatment with intravenous thrombolysis. The primary outcome measure was whether the decision to give thrombolysis was correct. Secondary outcomes were rate of thrombolytic use, 90-day functional outcomes, incidence of intracerebral hemorrhages, and technical observations.
Results: From December 2007 to October 2008, 54 patients were assessed, 27 of whom were randomized to each arm. Mean National Institutes of Health Stroke Scale score at presentation was 7.3 (SD 6.2) points. No consultations were aborted; however, technical problems (74%) were prevalent in the telemedicine arm. Overall, the correct treatment decision was established in 87% of the consultations. Both modalities, telephone (89% correct) and telemedicine (85% correct), performed well. Intravenous thrombolytic treatment was used in 30% of the telemedicine and telephone consultations. Good functional outcomes at 90 days were not significantly different. There were no statistically significant differences in mortality (4% in telemedicine and 11% in telephone) or rates of intracerebral hemorrhage (4% in telemedicine and 0% in telephone).
Conclusions: It is feasible to extend the original STRokE DOC trial protocol to a new state and establish an operational single-hub, multispoke rural hospital telestroke research network in Arizona. The trial was not designed to have sufficient power to detect a difference between the 2 consultative modes: telemedicine and telephone-only. Whether by telemedicine or telephone consultative modalities, there were appropriate treatment decisions, high rates of thrombolysis use, improved data collection, low rates of intracerebral hemorrhage, and equally favorable time requirements. The learning curve was steep for the hub and spoke personnel of the new telestroke network, as reflected by frequent technical problems. Overall, the results support the effectiveness of highly organized and structured stroke telemedicine networks for extending expert stroke care into rural remote communities lacking sufficient neurological expertise.
Conflict of interest statement
Conflicts of interest: We have no conflicts of interest.
References
-
- Joubert J, Prentice LF, Moulin T, Liaw ST, Joubert LB, Preux PM, Ware D, Medeiros de Bustos E, McLean A. Stroke in rural areas and small communities. Stroke. 2008;39:1920–1928. - PubMed
-
- Leira EC, Hess DC, Torner JC, Adams HP., Jr Rural-urban differences in acute stroke management practices: A modifiable disparity. Archives of Neurology. 2008;65:887–891. - PubMed
-
- Miley ML, Demaerschalk BM, Olmstead NL, Kiernan TE, Corday DA, Chikani V, Bobrow BJ. The state of emergency stroke resources and care in rural arizona: A platform for telemedicine. Telemedicine and e-Health. 2009;15:691–699. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical