Differences in N-glycan structures found on recombinant IgA1 and IgA2 produced in murine myeloma and CHO cell lines
- PMID: 20431350
- PMCID: PMC2881258
- DOI: 10.4161/mabs.2.3.11802
Differences in N-glycan structures found on recombinant IgA1 and IgA2 produced in murine myeloma and CHO cell lines
Abstract
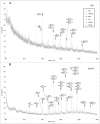
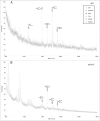
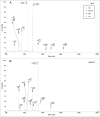
The development and production of recombinant monoclonal antibodies is well established. Although most of these are IgGs, there is also great interest in producing recombinant IgAs since this isotype plays a critical role in providing immunologic protection at mucosal surfaces. The choice of expression system for production of recombinant antibodies is crucial because they are glycoproteins containing at least one N-linked carbohydrate. These glycans have been shown to contribute to the stability, pharmacokinetics and biologic function of antibodies. We have produced recombinant human IgA1 and all three allotypes of IgA2 in murine myeloma and CHO cell lines to systematically characterize and compare the N-linked glycans. Recombinant IgAs produced in murine myelomas differ significantly from IgA found in humans in that they contain the highly immunogenic Galalpha(1,3)Gal epitope and N-glycolylneuraminic acid residues, indicating that murine myeloma is not the optimal expression system for the production of human IgA. In contrast, IgAs produced in CHO cells contained glycans that were more similar to those found on human IgA. Expression of IgA1 and IgA2 in Lec2 and Lec8 cell lines that are defective in glycan processing resulted in a less complex pool of N-glycans. In addition, the level of sialylation of rIgAs produced in murine and CHO cells was significantly lower than that previously reported for serum IgA1. These data underscore the importance of choosing the appropriate cell line for the production of glycoproteins with therapeutic potential.
Figures








References
-
- Stubbe H, Berdoz J, Kraehenbuhl JP, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J Immunol. 2000;164:1952–1960. - PubMed
-
- Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4:601–606. - PubMed
-
- Vidarsson G, van Der Pol WL, van Den Elsen JM, Vile H, Jansen M, Duijs J, et al. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J Immunol. 2001;166:6250–6256. - PubMed
-
- Stockmeyer B, Dechant M, van Egmond M, Tutt AL, Sundarapandiyan K, Graziano RF, et al. Triggering Fcalpha-receptor I (CD89) recruits neutrophils as effector cells for CD20-directed antibody therapy. J Immunol. 2000;165:5954–5961. - PubMed
-
- van Egmond M, van Spriel AB, Vermeulen H, Huls G, van Garderen E, van de Winkel JG. Enhancement of polymorphonuclear cell-mediated tumor cell killing on simultaneous engagement of FcgammaRI (CD64) and FcalphaRI (CD89) Cancer Res. 2001;61:4055–4060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
