Phase I/II, open-label trial of safety and immunogenicity of meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine in human immunodeficiency virus-infected adolescents
- PMID: 20431379
- PMCID: PMC2868314
- DOI: 10.1097/INF.0b013e3181c38f3b
Phase I/II, open-label trial of safety and immunogenicity of meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine in human immunodeficiency virus-infected adolescents
Abstract
Background: Quadrivalent meningococcal polysaccharide conjugate vaccine (MCV4) is routinely recommended for healthy youth in the United States, but there are no data about its use in HIV-infected people.
Methods: P1065 is a Phase I/II trial of MCV4 safety and immunogenicity in HIV-infected children and youth performed at 27 US sites of the IMPAACT network. All youth (11-24 years old) received 1 dose of open-label MCV4 at entry. Standardized questionnaires were used to evaluate safety. Baseline protective immunity was defined as rabbit serum bactericidal antibody (rSBA) titer > or = 1:128. Immunogenic response was defined as a > or = 4-fold rise in rSBA against each meningococcal serogroup. Multivariable logistic regression analysis was used to evaluate the association of demographic and clinical characteristics on immunogenic response to serogroup C.
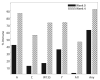
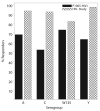
Results: Among 319 subjects who received MCV4, 10 (3.1%) reported immediate adverse events which were local and mild, and 7 (2.2%) experienced Grade > or = 3 adverse events, unrelated to vaccine. The 305 subjects with serologic data had a median age of 17 years and were 59% male, 50% Black, and 38% Latino. Subjects were stratified by entry CD4%: 12%, CD4 <15%; 40%, 15% to 24%; and 48%, > or = 25%. Baseline protective immunity varied by serogroup: A, 41%; C, 11%; W-135, 15%; Y, 35% The immunogenic response rates to serogroups A, C, W-135, and Y were 68%, 52%, 73%, and 63%, respectively. In multivariable logistic regression models, lower entry CD4%, higher entry viral load, and CDC Class B/C diagnosis were associated with significantly lower odds of response to serogroup C.
Conclusion: Many HIV-infected youth naturally acquire meningococcal immunity. MCV4 is safe and immunogenic in HIV-infected youth, but response rates are lower than in healthy youth, particularly for those with more advanced HIV clinical, immunologic, and virologic status.
Figures
References
-
- Recommendation from the Advisory Committee on Immunization Practices (ACIP) for Use of Quadrivalent Meningococcal Conjugate Vaccine (MCV4) in children aged 2–10 years at increased risk for invasive meningococcal disease. MMWR. 2007;56:1265–1266.
-
- CDC Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2005;54(RR-7):1–21. - PubMed
-
- Keyserling H, Papa T, Koranyi K, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–913. - PubMed
-
- CDC Guillain-Barré Syndrome among recipients of Menactra meningococcal conjugate vaccine—United States, June–July 2005. MMWR. 2005;54:1023–1025. - PubMed
-
- CDC Update: Guillain-Barré Syndrome among recipients of Menactra meningococcal conjugate vaccine-United States, June 2005–September 2006. MMWR. 2006;55:1120–1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- N01-DK-9-001/HHSN267200800001C/DK/NIDDK NIH HHS/United States
- HHSN272200800014C/AI/NIAID NIH HHS/United States
- AI068632/AI/NIAID NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5 U01 AI41110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials