Enzymatic deconstruction of xylan for biofuel production
- PMID: 20431716
- PMCID: PMC2860967
- DOI: 10.1111/j.1757-1707.2009.01004.x
Enzymatic deconstruction of xylan for biofuel production
Abstract
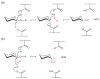
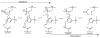
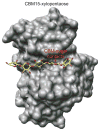
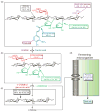
The combustion of fossil-derived fuels has a significant impact on atmospheric carbon dioxide (CO(2)) levels and correspondingly is an important contributor to anthropogenic global climate change. Plants have evolved photosynthetic mechanisms in which solar energy is used to fix CO(2) into carbohydrates. Thus, combustion of biofuels, derived from plant biomass, can be considered a potentially carbon neutral process. One of the major limitations for efficient conversion of plant biomass to biofuels is the recalcitrant nature of the plant cell wall, which is composed mostly of lignocellulosic materials (lignin, cellulose, and hemicellulose). The heteropolymer xylan represents the most abundant hemicellulosic polysaccharide and is composed primarily of xylose, arabinose, and glucuronic acid. Microbes have evolved a plethora of enzymatic strategies for hydrolyzing xylan into its constituent sugars for subsequent fermentation to biofuels. Therefore, microorganisms are considered an important source of biocatalysts in the emerging biofuel industry. To produce an optimized enzymatic cocktail for xylan deconstruction, it will be valuable to gain insight at the molecular level of the chemical linkages and the mechanisms by which these enzymes recognize their substrates and catalyze their reactions. Recent advances in genomics, proteomics, and structural biology have revolutionized our understanding of the microbial xylanolytic enzymes. This review focuses on current understanding of the molecular basis for substrate specificity and catalysis by enzymes involved in xylan deconstruction.
Figures

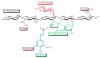
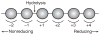


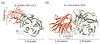

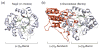
References
-
- Agbogbo FK, Coward-Kelly G. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnology Letters. 2008;30:1515–1524. - PubMed
-
- Antal MJ, Jr, Mok WS, Richards GN. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydrate Research. 1990;199:91–109. - PubMed
-
- Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology. 1992;23:257–270.
-
- Bayer EA, Lamed R, Himmel ME. The potential of cellulases and cellulosomes for cellulosic waste management. Current Opinion in Biotechnology. 2007;18:237–245. - PubMed
-
- Berrin JG, Juge N. Factors affecting xylanase functionality in the degradation of arabinoxylans. Biotechnology Letters. 2008;30:1139–1150. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
