Magnetic nanoparticles for imaging dendritic cells
- PMID: 20432309
- PMCID: PMC4888962
- DOI: 10.1002/mrm.22313
Magnetic nanoparticles for imaging dendritic cells
Abstract
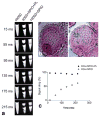
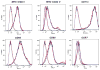
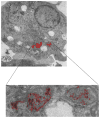
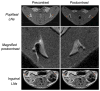
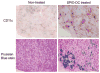
We report the development of superparamagnetic iron oxide (SPIOs) nanoparticles and investigate the migration of SPIO-labeled dendritic cells (DCs) in a syngeneic mouse model using magnetic resonance (MR) imaging. The size of the dextran-coated SPIO is roughly 30 nm, and the DCs are capable of independent uptake of these particles, although not at levels comparable to particle uptake in the presence of a transfecting reagent. On average, with the assistance of polylysine, the particles were efficiently delivered inside DCs within one hour of incubation. The SPIO particles occupy approximately 0.35% of cell surface and are equivalent to 34.6 pg of iron per cell. In vivo imaging demonstrated that the labeled cells migrated from the injection site in the footpad to the corresponding popliteal lymph node. The homing of labeled cells in the lymph nodes resulted in a signal drop of up to 79%. Furthermore, labeling DCs with SPIO particles did not compromise cell function, we demonstrated that SPIO-enhanced MR imaging can be used to track the migration of DCs effectively in vivo.
Figures


References
-
- Mohty M, Olive D, Gaugler B. Leukemic dendritic cells: potential for therapy and insights towards immune escape by leukemic blasts. Leukemia. 2002;16:2197–2204. - PubMed
-
- Ni K, O’Neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223–230. - PubMed
-
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. - PubMed
-
- Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

