The effect of charge-reversal amphiphile spacer composition on DNA and siRNA delivery
- PMID: 20433165
- PMCID: PMC3052776
- DOI: 10.1021/bc9005464
The effect of charge-reversal amphiphile spacer composition on DNA and siRNA delivery
Abstract
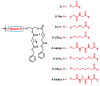
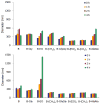
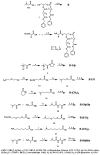
A series of charge-reversal amphiphiles with different spacers separating the headgroup from the hydrophobic chains are described for delivery of DNA and siRNA. Among them, the amphiphiles possessing a glycine spacer (e.g., B-GlyGly) showed effective DNA transfection in CHO and NIH 3T3 cells, as well as siRNA gene knockdown in HepG2 and UASMC cells. Ethidium bromide quenching assays revealed that DNA was released the fastest from the lipoplex of B-GlyGly in the presence of esterase. Also, X-ray diffraction results indicated that the DNA was located between the adjacent lipid bilayers in the lipoplex of B-GlyGly. These distinct features appear to be required for high transfection activity.
Figures





References
-
- Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. - PubMed
-
- Ewert KK, Ahmad A, Bouxsein NF, Evans HM, Safinya CR. Non-viral gene delivery with cationic liposome-DNA complexes. Meth Mol Biol. 2008;433:159–175. - PubMed
-
- Christine CC, Huang L. Recent advances in non-viral gene delivery. Adv Genetics. 2005;53:3–18. - PubMed
-
- Wolff JA, Rozema DB. Breaking the bonds: Non-viral vectors become chemically dynamic. Mol Therapy. 2008;16:8–15. - PubMed
-
- Reineke TM, Grinstaff MW. Designer materials for nucleic acid delivery. Mat Res Soc Bull. 2005;30:635–639.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

