The divalent metal ion in the active site of uteroferrin modulates substrate binding and catalysis
- PMID: 20433174
- PMCID: PMC2892236
- DOI: 10.1021/ja910583y
The divalent metal ion in the active site of uteroferrin modulates substrate binding and catalysis
Abstract
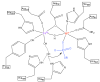
The purple acid phosphatases (PAP) are binuclear metallohydrolases that catalyze the hydrolysis of a broad range of phosphomonoester substrates. The mode of substrate binding during catalysis and the identity of the nucleophile is subject to debate. Here, we used native Fe(3+)-Fe(2+) pig PAP (uteroferrin; Uf) and its Fe(3+)-Mn(2+) derivative to investigate the effect of metal ion substitution on the mechanism of catalysis. Replacement of the Fe(2+) by Mn(2+) lowers the reactivity of Uf. However, using stopped-flow measurements it could be shown that this replacement facilitates approximately a ten-fold faster reaction between both substrate and inorganic phosphate with the chromophoric Fe(3+) site. These data also indicate that in both metal forms of Uf, phenyl phosphate hydrolysis occurs faster than formation of a mu-1,3 phosphate complex. The slower rate of interaction between substrate and the Fe(3+) site relative to catalysis suggests that the substrate is hydrolyzed while coordinated only to the divalent metal ion. The likely nucleophile is a water molecule in the second coordination sphere, activated by a hydroxide terminally coordinated to Fe(3+). The faster rates of interaction with the Fe(3+) site in the Fe(3+)-Mn(2+) derivative than the native Fe(3+)-Fe(2+) form are likely mediated via a hydrogen bond network connecting the first and second coordination spheres, and illustrate how the selection of metal ions may be important in fine-tuning the function of this enzyme.
Figures





References
-
- Sträter N, Lipscomb WN, Klabunde T, Krebs B. Angew. Chem., Int. Ed. Engl. 1996;35:2025–2055.
-
- Wilcox DE. Chem. Rev. 1996;96:2435–2458. - PubMed
-
- Mitić N, Smith SJ, Neves A, Guddat LW, Gahan LR, Schenk G. Chem. Rev. 2006;106:3338–3363. - PubMed
-
- Cox RS, Schenk G, Mitić N, Gahan LR, Hengge AC. J. Am. Chem. Soc. 2007;129:9550–9551. - PubMed
-
- Oddie GW, Schenk G, Angel NZ, Walsh N, Guddat LW, de Jersey J, Cassady AI, Hamilton SE, Hume DA. Bone. 2000;27:575–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

