Control of erythrocyte membrane-skeletal cohesion by the spectrin-membrane linkage
- PMID: 20433199
- PMCID: PMC3056085
- DOI: 10.1021/bi1003684
Control of erythrocyte membrane-skeletal cohesion by the spectrin-membrane linkage
Abstract
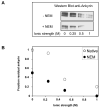
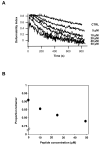
Spectrin tetramer is the major structural member of the membrane-associated skeletal network of red cells. We show here that disruption of the spectrin-ankyrin-band 3 link to the membrane leads to dissociation of a large proportion of the tetramers into dimers. Noncovalent perturbation of the linkage was induced by a peptide containing the ankyrin-binding site of the spectrin beta-chain, and covalent perturbation by treatment with the thiol reagent, N-ethylmaleimide (NEM). This reagent left the intrinsic self-association capacity of the spectrin dimers unaffected and disturbed only the ankyrin-band 3 interaction. The dissociation of spectrin tetramers on the membrane into functional dimers was confirmed by the binding of a spectrin peptide directed against the self-association sites. Dissociation of the tetramers resulted, we infer, from detachment of the proximal ends of the constituent dimers from the membrane, thereby reducing their proximity to one another and thus weakening their association. The measured affinity of the interaction of the peptides with the free dimer ends on the membrane permits an estimate of the equilibrium between intact and dissociated tetramers on the native membrane. This indicates that in the physiological state the equilibrium proportion of the dissociated tetramers may be as high as 5-10%. These findings enabled us to identify an additional important functional role for the spectrin-ankyrin-band 3 link in regulating spectrin self-association in the red cell membrane.
Figures



References
-
- Smith DK, Palek J. Sulfhydryl reagents induce altered spectrin self-association, skeletal instability, and increased thermal sensitivity of red cells. Blood. 1983;62:1190–1196. - PubMed
-
- Fischer TM, Haest CW, Stöhr M, Kamp D, Deuticke B. Selective alteration of erythrocyte deformabiliby by SH-reagents: evidence for an involvement of spectrin in membrane shear elasticity. Biochim. Biophys. Acta. 1978;510:270–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

