Viral nanoparticles as platforms for next-generation therapeutics and imaging devices
- PMID: 20433947
- PMCID: PMC2948632
- DOI: 10.1016/j.nano.2010.04.005
Viral nanoparticles as platforms for next-generation therapeutics and imaging devices
Abstract
Nanomaterials have been developed for potential applications in biomedicine, such as tissue-specific imaging and drug delivery. There are many different platforms under development, each with advantages and disadvantages, but viral nanoparticles (VNPs) are particularly attractive because they are naturally occurring nanomaterials, and as such they are both biocompatible and biodegradable. VNPs can be designed and engineered using both genetic and chemical protocols. The use of VNPs has evolved rapidly since their introduction 20 years ago, encompassing numerous chemistries and modification strategies that allow the functionalization of VNPs with imaging reagents, targeting ligands, and therapeutic molecules. This review discusses recent advances in the design of "smart" targeted VNPs for therapeutic and imaging applications.
From the clinical editor: This review focuses on viral nanoparticles, which are considered attractive naturally occurring nanomaterials due to their inherent biocompatibility and biodegradability. These can be used as imaging reagents, targeting ligands and therapeutic molecules.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



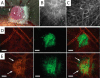
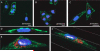
References
-
- Majoros IJ, Williams CR, Baker JR., Jr. Current dendrimer applications in cancer diagnosis and therapy. Curr Top Med Chem. 2008;8:1165–79. - PubMed
-
- Manchester M, Singh P. Virus-based nanoparticles (VNPs): platform technologies for diagnostic imaging. Adv Drug Deliv Rev. 2006;58:1505–22. - PubMed
-
- Soussan E, Cassel S, Blanzat M, Rico-Lattes I. Drug delivery by soft matter: matrix and vesicular carriers. Angewandte Chemie. 2009;48:274–88. - PubMed
-
- Xing Y, Rao J. Quantum dot bioconjugates for in vitro diagnostics & in vivo imaging. Cancer Biomark. 2008;4:307–19. - PubMed
-
- Portney NG, Ozkan M. Nano-oncology: drug delivery, imaging, and sensing. Anal Bioanal Chem. 2006;384:620–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

