Allele-specific expression of APC in adenomatous polyposis families
- PMID: 20434453
- PMCID: PMC2910837
- DOI: 10.1053/j.gastro.2010.04.047
Allele-specific expression of APC in adenomatous polyposis families
Abstract
Background & aims: Germline mutations in the APC gene cause of most cases of familial adenomatous polyposis (FAP) and a lesser proportion of attenuated FAP (AFAP). Systematic analysis of APC at the RNA level could provide insight into the pathogenicity of identified mutations and the molecular basis of FAP/AFAP in families without identifiable mutations. Here, we analyzed the prevalence of imbalances in the allelic expression of APC in polyposis families with germline mutations in the gene and without detectable mutations in APC and/or MUTYH.
Methods: Allele-specific expression (ASE) was determined by single nucleotide primer extension using an exon 11 polymorphism as an allele-specific marker. In total, 52 APC-mutation-positive (36 families) and 24 APC/MUTYH-mutation-negative (23 families) informative patients were analyzed. Seventy-six controls also were included.
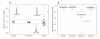
Results: Of the APC-mutation-positive families, most of those in whom the mutation was located before the last exon of the gene (12 of 14) had ASE imbalance, which is consistent with a mechanism of nonsense-mediated decay. Of the APC/MUTYH-mutation-negative families, 2 (9%) had ASE imbalance, which might cause the disease. Normal allele expression was restored shortly after lymphocytes were cultured with puromycin, supporting a 'nonsense-mediated' hypothesis.
Conclusions: ASE analysis might be used to determine the pathogenesis of some cases of FAP and AFAP in which APC mutations are not found. ASE also might be used to prioritize the order in which different areas of APC are tested. RNA-level studies are important for the molecular diagnosis of FAP.
Copyright (c) 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Lipton L, Tomlinson I. The genetics of FAP and FAP-like syndromes. Fam Cancer. 2006;5:221–6. - PubMed
-
- Heinimann K, Thompson A, Locher A, Furlanetto T, Bader E, Wolf A, Meier R, Walter K, Bauerfeind P, Marra G, Muller H, Foernzler D, Dobbie Z. Nontruncating APC germ-line mutations and mismatch repair deficiency play a minor role in APC mutation-negative polyposis. Cancer Res. 2001;61:7616–22. - PubMed
-
- Aretz S, Stienen D, Friedrichs N, Stemmler S, Uhlhaas S, Rahner N, Propping P, Friedl W. Somatic APC mosaicism: a frequent cause of familial adenomatous polyposis (FAP) Hum Mutat. 2007;28:985–92. - PubMed
-
- Castellsague E, Gonzalez S, Nadal M, Campos O, Guino E, Urioste M, Blanco I, Frebourg T, Capella G. Detection of APC gene deletions using quantitative multiplex PCR of short fluorescent fragments. Clin Chem. 2008;54:1132–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

