Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection
- PMID: 20434552
- PMCID: PMC2918737
- DOI: 10.1016/j.vaccine.2010.04.039
Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection
Abstract
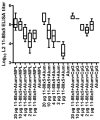
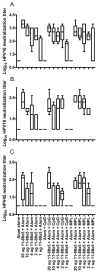
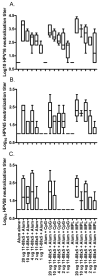
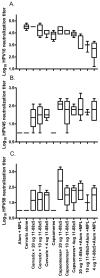
Immunization with L1 as pentavalent capsomeres or virus-like particles (VLPs) generates high and long-lived titers of neutralizing antibodies and protection primarily against the human papillomavirus (HPV) type from which the vaccine was derived. Conversely, vaccination with L2 minor capsid protein derived from multiple HPV types induces lower titer, but more broadly neutralizing and protective antibody responses. We combined the advantages of each protective antigen by immunization with titrated doses of multi-type L2 with either L1 capsomeres or VLP. We observed no significant interference between the L1 and L2 antibody response upon co-administration of L1 vaccines with multi-type L2 vaccines.
2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines.J Natl Cancer Inst. 2009 Jun 3;101(11):782-92. doi: 10.1093/jnci/djp106. Epub 2009 May 26. J Natl Cancer Inst. 2009. PMID: 19470949 Free PMC article.
-
Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles.J Virol. 2016 Jun 24;90(14):6314-25. doi: 10.1128/JVI.00449-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147749 Free PMC article.
-
Durable immunity to oncogenic human papillomaviruses elicited by adjuvanted recombinant Adeno-associated virus-like particle immunogen displaying L2 17-36 epitopes.Vaccine. 2015 Oct 13;33(42):5553-5563. doi: 10.1016/j.vaccine.2015.09.005. Epub 2015 Sep 15. Vaccine. 2015. PMID: 26382603 Free PMC article.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Papillomavirus-like particle vaccines.J Natl Cancer Inst Monogr. 2001;(28):50-4. doi: 10.1093/oxfordjournals.jncimonographs.a024258. J Natl Cancer Inst Monogr. 2001. PMID: 11158207 Review.
Cited by
-
Recent advancements in combination subunit vaccine development.Hum Vaccin Immunother. 2017 Jan 2;13(1):180-185. doi: 10.1080/21645515.2016.1229719. Hum Vaccin Immunother. 2017. PMID: 27649319 Free PMC article.
-
Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs.Front Immunol. 2020 Jun 9;11:1100. doi: 10.3389/fimmu.2020.01100. eCollection 2020. Front Immunol. 2020. PMID: 32582186 Free PMC article. Review.
-
Branched-linear and agglomerate protein polymers as vaccine platforms.Biomaterials. 2014 Sep;35(29):8427-38. doi: 10.1016/j.biomaterials.2014.06.021. Epub 2014 Jun 28. Biomaterials. 2014. PMID: 24985736 Free PMC article.
-
Identification of Broad-Genotype HPV L2 Neutralization Site for Pan-HPV Vaccine Development by a Cross-Neutralizing Antibody.PLoS One. 2015 Apr 23;10(4):e0123944. doi: 10.1371/journal.pone.0123944. eCollection 2015. PLoS One. 2015. PMID: 25905781 Free PMC article.
-
New Perspectives in Therapeutic Vaccines for HPV: A Critical Review.Medicina (Kaunas). 2022 Jun 28;58(7):860. doi: 10.3390/medicina58070860. Medicina (Kaunas). 2022. PMID: 35888579 Free PMC article. Review.
References
-
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006 Aug 21;24( Suppl 3):S11–25. - PubMed
-
- Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004 Aug 20;111(2):278–85. - PubMed
-
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

