Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection
- PMID: 20434552
- PMCID: PMC2918737
- DOI: 10.1016/j.vaccine.2010.04.039
Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection
Abstract
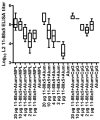
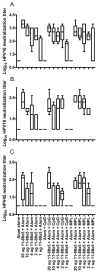
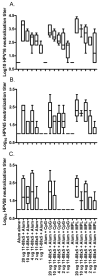
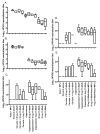
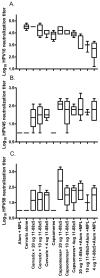
Immunization with L1 as pentavalent capsomeres or virus-like particles (VLPs) generates high and long-lived titers of neutralizing antibodies and protection primarily against the human papillomavirus (HPV) type from which the vaccine was derived. Conversely, vaccination with L2 minor capsid protein derived from multiple HPV types induces lower titer, but more broadly neutralizing and protective antibody responses. We combined the advantages of each protective antigen by immunization with titrated doses of multi-type L2 with either L1 capsomeres or VLP. We observed no significant interference between the L1 and L2 antibody response upon co-administration of L1 vaccines with multi-type L2 vaccines.
2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

References
-
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006 Aug 21;24( Suppl 3):S11–25. - PubMed
-
- Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004 Aug 20;111(2):278–85. - PubMed
-
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

