Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7
- PMID: 20434554
- PMCID: PMC2881612
- DOI: 10.1016/j.vaccine.2010.04.049
Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7
Abstract
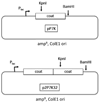
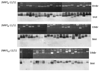
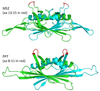
The immunogenicity of an antigen can be dramatically increased by displaying it in a dense, multivalent context, such as on the surface of a virus or virus-like particle (VLP). Here we describe a highly versatile VLP platform for peptide display based on VLPs of the RNA bacteriophage PP7. We show that this platform can be used for the engineered display of specific peptide sequences as well as for the construction of random peptide libraries. Peptides representing the FLAG epitope, the V3 loop of HIV gp120, and a broadly cross-type neutralizing epitope from L2, the minor capsid protein of Human Papillomavirus type 16 (HPV16), were inserted into an exposed surface loop of a form of PP7 coat protein in which the two identical polypeptides of coat were fused together to form a single-chain dimer. The recombinant proteins assembled into VLPs, displayed these peptides on their surfaces, and induced high-titer antibody responses. The single-chain dimer was also highly tolerant of random 6-, 8-, and 10-amino acid insertions. PP7 VLPs displaying the HPV16 L2 epitope generated robust anti-HPV16 L2 serum antibodies after intramuscular injection that protected mice from genital infection with HPV16 pseudovirus as well as a heterologous HPV pseudovirus type, HPV45. Thus, PP7 VLPs are well-suited for the display of a wide diversity of peptides in a highly immunogenic format.
Figures


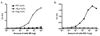


References
-
- Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007 Jun;6(3):381–390. - PubMed
-
- Spohn G, Bachmann MF. Exploiting viral properties for the rational design of modern vaccines. Expert Rev Vaccines. 2008 Feb;7(1):43–54. - PubMed
-
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children.Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997 Jun 26;336(26):1855–1859. - PubMed
-
- Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009 Dec 12;374(9706):1975–1985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

