Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome
- PMID: 20434582
- PMCID: PMC4074088
- DOI: 10.1016/j.micinf.2010.04.008
Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome
Abstract
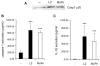
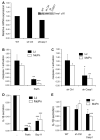
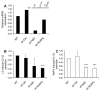
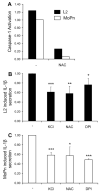
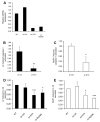
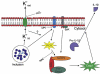
Chlamydia trachomatis infections represent the leading cause of bacterial sexually-transmitted disease in the United States and can cause serious tissue damage leading to infertility and ectopic pregnancies in women. Inflammation and hence the innate immune response to chlamydial infection contributes significantly to tissue damage, particularly by secreting proinflammatory cytokines such as interleukin (IL)-1beta from monocytes, macrophages and dendritic cells. Here we demonstrate that C. trachomatis or Chlamydia muridarum infection of a monocytic cell line leads to caspase-1 activation and IL-1beta secretion through a process requiring the NLRP3 inflammasome. Thus, secretion of IL-1beta decreased significantly when cells were depleted of NLRP3 or treated with the anti-inflammatory inhibitors parthenolide or Bay 11-7082, which inhibit inflammasomes and the transcription factor NF-kappaB. As for other infections causing NRLP3 inflammasome assembly, caspase-1 activation in monocytes is triggered by potassium efflux and reactive oxygen species production. However, anti-oxidants inhibited IL-1beta secretion only partially. Atypically for a bacterial infection, caspase-1 activation during chlamydial infection also involves partially the spleen tyrosine kinase (Syk), which is usually associated with a pathogen recognition receptor for fungal pathogens. Secretion of IL-1beta during infection by many bacteria requires both microbial products from the pathogen and an exogenous danger signal, but chlamydial infection provides both the pathogen-associated molecular patterns and danger signals necessary for IL-1beta synthesis and its secretion from human monocytes. Use of inhibitors that target the inflammasome in animals should therefore dampen inflammation during chlamydial infection.
Copyright 2010 Elsevier Masson SAS. All rights reserved.
Figures
References
-
- Gerbase AC, Rowley JT, Mertens TE. Global epidemiology of sexually transmitted diseases. Lancet. 1998;351:2–4. - PubMed
-
- Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–2236. - PubMed
-
- Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. ASM Press; Washington, D.C: 1999. pp. 139–169.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

