Frontal cortex and the discovery of abstract action rules
- PMID: 20435006
- PMCID: PMC2990347
- DOI: 10.1016/j.neuron.2010.03.025
Frontal cortex and the discovery of abstract action rules
Abstract
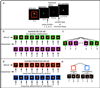
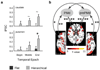
Although we often encounter circumstances with which we have no prior experience, we rapidly learn how to behave in these novel situations. Such adaptive behavior relies on abstract behavioral rules that are generalizable, rather than concrete rules mapping specific cues to specific responses. Although the frontal cortex is known to support concrete rule learning, less well understood are the neural mechanisms supporting the acquisition of abstract rules. Here, we use a reinforcement learning paradigm to demonstrate that more anterior regions along the rostro-caudal axis of frontal cortex support rule learning at higher levels of abstraction. Moreover, these results indicate that when humans confront new rule learning problems, this rostro-caudal division of labor supports the search for relationships between context and action at multiple levels of abstraction simultaneously.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. - PubMed
-
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. - PubMed
-
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

