Large-scale binding of α-crystallin to cell membranes of aged normal human lenses: a phenomenon that can be induced by mild thermal stress
- PMID: 20435594
- PMCID: PMC3066623
- DOI: 10.1167/iovs.10-5261
Large-scale binding of α-crystallin to cell membranes of aged normal human lenses: a phenomenon that can be induced by mild thermal stress
Abstract
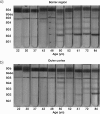
Purpose: With age, large amounts of crystallins become associated with fiber cell membranes in the human lens nucleus, and it has been proposed that this binding of protein may lead to the obstruction of membrane pores and the onset of a barrier to diffusion. This study focused on membrane binding within the barrier region and the outermost lens cortex.
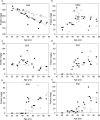
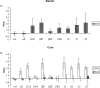
Methods: Human lenses across the age range were used, and the interaction of crystallins with membranes was examined using sucrose density gradient centrifugation, two-dimensional gel electrophoresis, and amine-reactive isobaric tagging technology. Lipids were quantified using shotgun lipidemics.
Results: Binding of proteins to cell membranes in the barrier region was found to be different from that in the lens nucleus because in the barrier and outer cortical regions, only one high-density band formed. Most of the membrane-associated protein in this high-density band was α-crystallin. Mild thermal stress of intact young lenses led to pronounced membrane binding of proteins and yielded a sucrose density pattern in all lens regions that appeared to be identical with that from older lenses.
Conclusions: α-Crystallin is the major protein that binds to cell membranes in the barrier region of lenses after middle age. Exposure of young human lenses to mild thermal stress results in large-scale binding of α-crystallin to cell membranes. The density gradient profiles of such heated lenses appear to be indistinguishable from those of older normal lenses. The data support the hypothesis that temperature may be a factor responsible for age-related changes to the human lens.
Figures




References
-
- Garland DL, Duglas TY, Jimenez AJ, et al. The nucleus of the human lens: demonstration of a highly characteristic protein pattern by two-dimensional electrophoresis and introduction of a new method of lens dissection. Exp Eye Res. 1996;62:285–291 - PubMed
-
- Kuszak JR. The development of lens sutures. Prog Retin Eye Res. 1995;14:567–591
-
- Cheng R, Feng Q, Argirov OK, et al. Structure elucidation of a novel yellow chromophore from human lens protein. J Biol Chem. 2004;279:45441–45449 - PubMed
-
- Lampi KJ, Ma Z, Hanson SRA, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

