Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells
- PMID: 20435848
- PMCID: PMC2904138
- DOI: 10.1152/ajpheart.00431.2009
Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells
Abstract
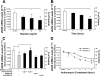
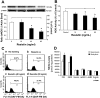
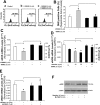
Resistin is a newly discovered adipocyte-derived cytokine that may play an important role in insulin resistance, diabetes, adipogenesis, inflammation, and cardiovascular disease. However, it is largely unknown whether resistin impairs endothelial functions by affecting the endothelial nitric oxide synthase (eNOS) system. In this study, we determined the effect of human recombinant resistin protein on eNOS expression and regulation in human coronary artery endothelial cells (HCAECs). When cells were treated with clinically relevant concentrations of resistin (40 or 80 ng/ml) for 24 h, the levels of eNOS mRNA, protein, and activity and eNOS mRNA stability were significantly reduced. Cellular nitric oxide levels were also decreased. In addition, the cellular levels of reactive oxygen species (ROS), including superoxide anion, were significantly increased in resistin-treated HCAECs. Mitochondrial membrane potential and the activities of catalase and superoxide dismutase were reduced. Three antioxidants, seleno-L-methionine, ginsenoside Rb1, and MnTBAP (superoxide dismutase mimetic), effectively blocked resistin-induced eNOS downregulation. Meanwhile, resistin activated the mitogen-activated protein kinases p38 and c-Jun NH(2)-terminal kinase (JNK), and the specific p38 inhibitor SB-239063 effectively blocked resistin-induced ROS production and eNOS downregulation. Furthermore, immunoreactivity of resistin was increased in atherosclerotic regions of human aorta and carotid arteries. Thus resistin directly induces eNOS downregulation through overproduction of ROS and activation of p38 and JNK in HCAECs. Resistin-induced mitochondrial dysfunction and imbalance in cellular redox enzymes may be the underlying mechanisms of oxidative stress.
Figures

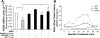

References
-
- Allan CB, Lacourciere GM, Stadtman TC. Responsiveness of selenoproteins to dietary selenium. Annu Rev Nutr 19: 1–16, 1999 - PubMed
-
- Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, Awano K, Kobayashi K, Maeda K, Hata K, Shinke T, Kobayashi S, Hirata K, Kawashima S, Itabe H, Hayashi Y, Imajoh-Ohmi S, Itoh H, Yokoyama M. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 22: 1838–1844, 2002 - PubMed
-
- Battin EE, Perron NR, Brumaghim JL. The central role of metal coordination in selenium antioxidant activity. Inorg Chem 45: 499–501, 2006 - PubMed
-
- Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, Zbinden S, Clavijo LC, Jang GJ, Andrews JA, Zhu J, Epstein SE. The potential role of resistin in atherogenesis. Atherosclerosis 182: 241–248, 2005 - PubMed
-
- Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr Hypertens Rep 10: 32–38, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

