Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement
- PMID: 20435900
- PMCID: PMC2879738
- DOI: 10.1105/tpc.109.069666
Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement
Abstract
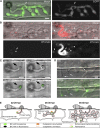
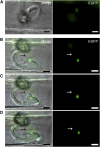
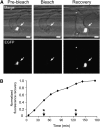
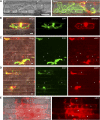
Knowledge remains limited about how fungal pathogens that colonize living plant cells translocate effector proteins inside host cells to regulate cellular processes and neutralize defense responses. To cause the globally important rice blast disease, specialized invasive hyphae (IH) invade successive living rice (Oryza sativa) cells while enclosed in host-derived extrainvasive hyphal membrane. Using live-cell imaging, we identified a highly localized structure, the biotrophic interfacial complex (BIC), which accumulates fluorescently labeled effectors secreted by IH. In each newly entered rice cell, effectors were first secreted into BICs at the tips of the initially filamentous hyphae in the cell. These tip BICs were left behind beside the first-differentiated bulbous IH cells as the fungus continued to colonize the host cell. Fluorescence recovery after photobleaching experiments showed that the effector protein PWL2 (for prevents pathogenicity toward weeping lovegrass [Eragrostis curvula]) continued to accumulate in BICs after IH were growing elsewhere. PWL2 and BAS1 (for biotrophy-associated secreted protein 1), BIC-localized secreted proteins, were translocated into the rice cytoplasm. By contrast, BAS4, which uniformly outlines the IH, was not translocated into the host cytoplasm. Fluorescent PWL2 and BAS1 proteins that reached the rice cytoplasm moved into uninvaded neighbors, presumably preparing host cells before invasion. We report robust assays for elucidating the molecular mechanisms that underpin effector secretion into BICs, translocation to the rice cytoplasm, and cell-to-cell movement in rice.
Figures


Comment in
-
Blast effector proteins may pave the way for hyphal invasion.Plant Cell. 2010 Apr;22(4):996. doi: 10.1105/tpc.110.220413. Epub 2010 Apr 30. Plant Cell. 2010. PMID: 20435905 Free PMC article. No abstract available.
-
Magnaporthe effectors on the move.Nat Rev Microbiol. 2010 Jul;8(7):466. doi: 10.1038/nrmicro2394. Nat Rev Microbiol. 2010. PMID: 21394961 No abstract available.
References
-
- Ai H.-W., Shaner N.C., Cheng Z., Tsien R.Y., Campbell R.E. (2007). Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry 46: 5904–5910 - PubMed
-
- Ballini E., Morel J.-B., Droc G., Price A., Courtois B., Notteghem J.-L., Tharreau D. (2008). A genome-wide meta-analysis of rice blast resistance genes and quantitative trait loci provides new insights into partial and complete resistance. Mol. Plant Microbe Interact. 21: 859–868 - PubMed
-
- Berruyer R., Poussier S., Kankanala P., Mosquera G., Valent B. (2006). Quantitative and qualitative influence of inoculation methods on in planta growth of rice blast fungus. Phytopathology 96: 346–355 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

