Dronedarone in patients with congestive heart failure: insights from ATHENA
- PMID: 20436046
- PMCID: PMC2903712
- DOI: 10.1093/eurheartj/ehq113
Dronedarone in patients with congestive heart failure: insights from ATHENA
Abstract
Aims: Dronedarone is a new multichannel blocking antiarrhythmic drug for treatment of atrial fibrillation (AF). In patients with recently decompensated congestive heart failure (CHF) and depressed LV function, the drug was associated with excess mortality compared with a placebo group. The present study aimed to analyse in detail the effects of dronedarone on mortality and morbidity in AF patients CHF.
Methods and results: We performed a post hoc analysis of ATHENA, a large placebo-controlled outcome trial in 4628 patients with paroxysmal or persistent AF, to evaluate the relationship between clinical outcomes and dronedarone therapy in patients with stable CHF. The primary outcome was time to first cardiovascular (CV) hospitalization or death. There were 209 patients with NYHA class II/III CHF and a left ventricular ejection fraction < or =0.40 at baseline (114 placebo, 95 dronedarone patients). A primary outcome event occurred in 59/114 placebo patients compared with 42/95 dronedarone patients [hazard ratio (HR) 0.78, 95% CI = 0.52-1.16]. Twenty of 114 placebo patients and 12/95 dronedarone patients died during the study (HR 0.71, 95% CI = 0.34-1.44). Fifty-four placebo and 42 dronedarone patients were hospitalized for an intermittent episode of NYHA class IV CHF (HR = 0.78, 95% CI = 0.52-1.17).
Conclusion: In this post-hoc analysis of ATHENA patients with AF and stable CHF, dronedarone did not increase mortality and showed a reduction of CV hospitalization or death similar to the overall population. However, in the light of the ANtiarrhythmic trial with DROnedarone in Moderate to severe CHF Evaluating morbidity DecreAse study, dronedarone should be contraindicated in patients with NYHA class IV or unstable NYHA classes II and III CHF.
Trial registration: ClinicalTrials.gov NCT00174785.
Figures

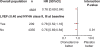

References
-
- Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey P, Capucci A, Radzik D, Aliot E, Hohnloser SH. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999. doi:10.1056/NEJMoa054686. - DOI - PubMed
-
- Davy JM, Herold M, Hoglund C, Timmermans A, Alings A, Radzik D, Van Kempen L ERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J. 2008;156:527. e1-9. doi:10.1016/j.ahj.2008.06.010. - DOI - PubMed
-
- Hohnloser SH, Crijns HJGM, van Eickels M, Gaudin C, Page R, Torp-Pedersen C, Connolly S. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678. doi:10.1056/NEJMoa0803778. - DOI - PubMed
-
- Kober L, Torp-Pedersen C, McMurray JJV, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J for the dronedarone study group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. doi:10.1056/NEJMoa0800456. - DOI - PubMed
-
- Hohnloser SH, Connolly SJ, Crijns HJ, Page RL, Seiz W, Torp-Petersen C. Rationale and design of ATHENA: a placebo-controlled, double-blind, parallel arm trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. J Cardiovasc Electrophysiol. 2008;19:69–73. doi:10.1111/j.1540-8167.2007.01016.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

