C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog
- PMID: 20436480
- PMCID: PMC2882063
- DOI: 10.1038/nn.2540
C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog
Abstract
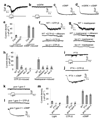
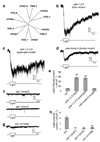
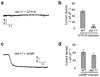
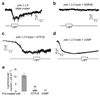
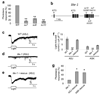
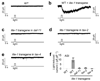
The eyeless animal C. elegans is able to sense light and engages in phototaxis behavior that is mediated by photoreceptor cells. However, the molecular and cellular mechanisms underlying phototransduction in C. elegans remain largely unclear. By recording the photoreceptor neuron ASJ in wild-type and various mutant worms, we found that phototransduction in ASJ is a G protein-mediated process and requires membrane-associated guanylate cyclases, but not typical phosphodiesterases. In addition, we found that C. elegans phototransduction requires LITE-1, a candidate photoreceptor protein known to be a member of the invertebrate taste receptor family. Our genetic, pharmacological and electrophysiological data suggest a model in which LITE-1 transduces light signals in ASJ via G protein signaling, which leads to upregulation of the second messenger cGMP, followed by opening of cGMP-sensitive CNG channels and stimulation of photoreceptor cells. Our results identify a phototransduction cascade in C. elegans and implicate the function of a 'taste receptor' in phototransduction.
Figures

References
-
- Xiong WH, Solessio EC, Yau KW. An unusual cGMP pathway underlying depolarizing light response of the vertebrate parietal-eye photoreceptor. Nature neuroscience. 1998;1:359–365. - PubMed
-
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. - PubMed
-
- Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

