Memory CD4+ T cells induce innate responses independently of pathogen
- PMID: 20436484
- PMCID: PMC2927232
- DOI: 10.1038/nm.2142
Memory CD4+ T cells induce innate responses independently of pathogen
Abstract
Inflammation induced by recognition of pathogen-associated molecular patterns markedly affects subsequent adaptive responses. We asked whether the adaptive immune system can also affect the character and magnitude of innate inflammatory responses. We found that the response of memory, but not naive, CD4(+) T cells enhances production of multiple innate inflammatory cytokines and chemokines (IICs) in the lung and that, during influenza infection, this leads to early control of virus. Memory CD4(+) T cell-induced IICs and viral control require cognate antigen recognition and are optimal when memory cells are either T helper type 1 (T(H)1) or T(H)17 polarized but are independent of interferon-gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha) production and do not require activation of conserved pathogen recognition pathways. This represents a previously undescribed mechanism by which memory CD4(+) T cells induce an early innate response that enhances immune protection against pathogens.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
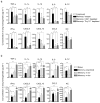

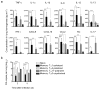
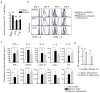

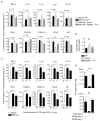
Comment in
-
Innate immunity: Experience helps.Nat Rev Immunol. 2010 Jun;10(6):380. doi: 10.1038/nri2790. Nat Rev Immunol. 2010. PMID: 20514668 No abstract available.
References
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–216. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. - PubMed
-
- Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. - PubMed
-
- Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science (New York, N Y) 2001;293:253–256. - PubMed
-
- Powell TJ, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

