Small molecules discovered in a pathway screen target the Rho pathway in cytokinesis
- PMID: 20436488
- PMCID: PMC2873065
- DOI: 10.1038/nchembio.363
Small molecules discovered in a pathway screen target the Rho pathway in cytokinesis
Abstract
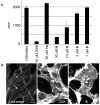
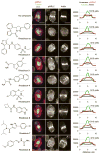
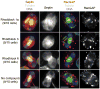
We report the discovery of small molecules that target the Rho pathway, which is a central regulator of cytokinesis--the final step in cell division. We have developed a way of targeting a small molecule screen toward a specific pathway, which should be widely applicable to the investigation of any signaling pathway. In a chemical genetic variant of a classical modifier screen, we used RNA interference (RNAi) to sensitize cells and identified small molecules that suppressed or enhanced the RNAi phenotype. We discovered promising candidate molecules, which we named Rhodblock, and we identified the target of Rhodblock as Rho kinase. Several Rhodblocks inhibited one function of the Rho pathway in cells: the correct localization of phosphorylated myosin light chain during cytokinesis. Rhodblocks differentially perturb Rho pathway proteins in cells and can be used to dissect the mechanism of the Rho pathway during cytokinesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Giving Rho(d) directions.Nat Chem Biol. 2010 Jun;6(6):397-8. doi: 10.1038/nchembio.373. Nat Chem Biol. 2010. PMID: 20479746 No abstract available.
References
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. - PubMed
-
- Gomez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27:602–613. - PubMed
-
- Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. - PubMed
-
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

