Critical evaluation of gamma-irradiated serum used as feeder in the culture and demonstration of putative nanobacteria and calcifying nanoparticles
- PMID: 20436679
- PMCID: PMC2859944
- DOI: 10.1371/journal.pone.0010343
Critical evaluation of gamma-irradiated serum used as feeder in the culture and demonstration of putative nanobacteria and calcifying nanoparticles
Abstract
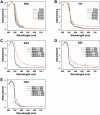
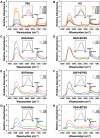
The culture and demonstration of putative nanobacteria (NB) and calcifying nanoparticles (CNP) from human and animal tissues has relied primarily on the use of a culture supplement consisting of FBS that had been gamma-irradiated at a dose of 30 kGy (gamma-FBS). The use of gamma-FBS is based on the assumption that this sterilized fluid has been rid entirely of any residual NB/CNP, while it continues to promote the slow growth in culture of NB/CNP from human/animal tissues. We show here that gamma-irradiation (5-50 kGy) produces extensive dose-dependent serum protein breakdown as demonstrated through UV and visible light spectrophotometry, fluorometry, Fourier-transformed infrared spectroscopy, and gel electrophoresis. Yet, both gamma-FBS and gamma-irradiated human serum (gamma-HS) produce NB/CNP in cell culture conditions that are morphologically and chemically indistinguishable from their normal serum counterparts. Contrary to earlier claims, gamma-FBS does not enhance the formation of NB/CNP from several human body fluids (saliva, urine, ascites, and synovial fluid) tested. In the presence of additional precipitating ions, both gamma-irradiated serum (FBS and HS) and gamma-irradiated proteins (albumin and fetuin-A) retain the inherent dual NB inhibitory and seeding capabilities seen also with their untreated counterparts. By gel electrophoresis, the particles formed from both gamma-FBS and gamma-HS are seen to have assimilated into their scaffold the same smeared protein profiles found in the gamma-irradiated sera. However, their protein compositions as identified by proteomics are virtually identical to those seen with particles formed from untreated serum. Moreover, particles derived from human fluids and cultured in the presence of gamma-FBS contain proteins derived from both gamma-FBS and the human fluid under investigation-a confusing and unprecedented scenario indicating that these particles harbor proteins from both the host tissue and the FBS used as feeder. Thus, the NB/CNP described in the literature clearly bear hybrid protein compositions belonging to different species. We conclude that there is no basis to justify the use of gamma-FBS as a feeder for the growth and demonstration of NB/CNP or any NB-like particles in culture. Moreover, our results call into question the validity of the entire body of literature accumulated to date on NB and CNP.
Conflict of interest statement
Figures











References
-
- Young JD, Martel J. The rise and fall of nanobacteria. Sci Am. 2010;302:52–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

