Isoform-specific upregulation of palladin in human and murine pancreas tumors
- PMID: 20436683
- PMCID: PMC2859948
- DOI: 10.1371/journal.pone.0010347
Isoform-specific upregulation of palladin in human and murine pancreas tumors
Abstract
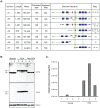
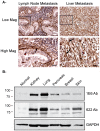
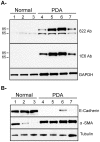
Pancreatic ductal adenocarcinoma (PDA) is a lethal disease with a characteristic pattern of early metastasis, which is driving a search for biomarkers that can be used to detect the cancer at an early stage. Recently, the actin-associated protein palladin was identified as a candidate biomarker when it was shown that palladin is mutated in a rare inherited form of PDA, and overexpressed in many sporadic pancreas tumors and premalignant precursors. In this study, we analyzed the expression of palladin isoforms in murine and human PDA and explored palladin's potential use in diagnosing PDA. We performed immunohistochemistry and immunoblot analyses on patient samples and tumor-derived cells using an isoform-selective monoclonal antibody and a pan-palladin polyclonal antibody. Immunoblot and real-time quantitative reverse transcription-PCR were used to quantify palladin mRNA levels in human samples. We show that there are two major palladin isoforms expressed in pancreas: 65 and 85-90 kDa. The 65 kDa isoform is expressed in both normal and neoplastic ductal epithelial cells. The 85-90 kDa palladin isoform is highly overexpressed in tumor-associated fibroblasts (TAFs) in both primary and metastatic tumors compared to normal pancreas, in samples obtained from either human patients or genetically engineered mice. In tumor-derived cultured cells, expression of palladin isoforms follows cell-type specific patterns, with the 85-90 kDa isoform in TAFs, and the 65 kDa isoform predominating in normal and neoplastic epithelial cells. These results suggest that upregulation of 85-90 kDa palladin isoform may play a role in the establishment of the TAF phenotype, and thus in the formation of a desmoplastic tumor microenvironment. Thus, palladin may have a potential use in the early diagnosis of PDA and may have much broader significance in understanding metastatic behavior.
Conflict of interest statement
Figures



References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. - PubMed
-
- Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK0606994/DK/NIDDK NIH HHS/United States
- R01 CA129357/CA/NCI NIH HHS/United States
- P50-CA10699/CA/NCI NIH HHS/United States
- T32 CA009688/CA/NCI NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- CA114028/CA/NCI NIH HHS/United States
- K08 CA138912/CA/NCI NIH HHS/United States
- R21 CA155650/CA/NCI NIH HHS/United States
- CA15704/CA/NCI NIH HHS/United States
- R01 GM081505/GM/NIGMS NIH HHS/United States
- P50-CA106991/CA/NCI NIH HHS/United States
- CA129357/CA/NCI NIH HHS/United States
- P50 CA106991/CA/NCI NIH HHS/United States
- K08 CA098240/CA/NCI NIH HHS/United States
- MOP-36332/CAPMC/ CIHR/Canada
- P30 CA015704/CA/NCI NIH HHS/United States
- K08 CA114028/CA/NCI NIH HHS/United States
- R01 CA113451/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

