Specific immunotherapy of experimental myasthenia gravis by a novel mechanism
- PMID: 20437579
- PMCID: PMC2864953
- DOI: 10.1002/ana.21901
Specific immunotherapy of experimental myasthenia gravis by a novel mechanism
Abstract
Objective: Myasthenia gravis (MG) and its animal model, experimental autoimmune myasthenia gravis (EAMG), are antibody (Ab)-mediated autoimmune diseases, in which autoantibodies bind to and cause loss of muscle nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction. To develop a specific immunotherapy of MG, we treated rats with ongoing EAMG by intraperitoneal injection of bacterially-expressed human muscle AChR constructs.
Methods: Rats with ongoing EAMG received intraperitoneal treatment with the constructs weekly for 5 weeks beginning after the acute phase. Autoantibody concentration, subclassification, and specificity were analyzed to address the underlying therapeutic mechanism.
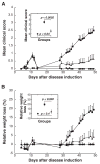
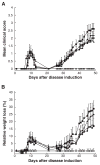
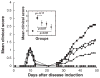
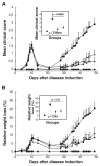
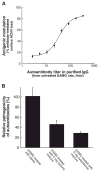
Results: EAMG was specifically suppressed by diverting autoantibody production away from pathologically relevant specificities directed at epitopes on the extracellular surface of muscle AChRs toward pathologically irrelevant epitopes on the cytoplasmic domain. A mixture of subunit cytoplasmic domains was more effective than a mixture containing both extracellular and cytoplasmic domains or than only the extracellular domain of alpha1 subunits.
Interpretation: Therapy using only cytoplasmic domains, which lack pathologically relevant epitopes, avoids the potential liability of boosting the pathological response. Use of a mixture of bacterially-expressed human muscle AChR cytoplasmic domains for antigen-specific immunosuppression of myasthenia gravis has the potential to be specific, robust, and safe.
Figures

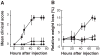

Comment in
-
Zero tolerance (to acetylcholine receptor) and ways to overcome it.Ann Neurol. 2010 Apr;67(4):422-4. doi: 10.1002/ana.22025. Ann Neurol. 2010. PMID: 20437576 No abstract available.
Similar articles
-
Myasthenogenicity of the main immunogenic region.Ann N Y Acad Sci. 2012 Dec;1274(1):9-13. doi: 10.1111/j.1749-6632.2012.06766.x. Ann N Y Acad Sci. 2012. PMID: 23252892 Free PMC article.
-
Acetylcholine receptor-specific immunosuppressive therapy of experimental autoimmune myasthenia gravis and myasthenia gravis.Ann N Y Acad Sci. 2018 Feb;1413(1):76-81. doi: 10.1111/nyas.13550. Epub 2018 Jan 29. Ann N Y Acad Sci. 2018. PMID: 29377167 Review.
-
Antigen-specific immunotherapeutic vaccine for experimental autoimmune myasthenia gravis.J Immunol. 2014 Nov 15;193(10):5044-55. doi: 10.4049/jimmunol.1401392. Epub 2014 Oct 6. J Immunol. 2014. PMID: 25288571 Free PMC article.
-
Myasthenia gravis and the tops and bottoms of AChRs: antigenic structure of the MIR and specific immunosuppression of EAMG using AChR cytoplasmic domains.Ann N Y Acad Sci. 2008;1132:29-41. doi: 10.1196/annals.1405.007. Ann N Y Acad Sci. 2008. PMID: 18567851 Free PMC article.
-
AChR-specific immunosuppressive therapy of myasthenia gravis.Biochem Pharmacol. 2015 Oct 15;97(4):609-619. doi: 10.1016/j.bcp.2015.07.011. Epub 2015 Jul 26. Biochem Pharmacol. 2015. PMID: 26215875 Review.
Cited by
-
Advances in autoimmune myasthenia gravis management.Expert Rev Neurother. 2018 Jul;18(7):573-588. doi: 10.1080/14737175.2018.1491310. Epub 2018 Jul 4. Expert Rev Neurother. 2018. PMID: 29932785 Free PMC article. Review.
-
A comparison of epitope repertoires associated with myasthenia gravis in humans and nonhuman hosts.Autoimmune Dis. 2012;2012:403915. doi: 10.1155/2012/403915. Epub 2012 Dec 2. Autoimmune Dis. 2012. PMID: 23243503 Free PMC article.
-
Myasthenia gravis: the future is here.J Clin Invest. 2024 Jun 17;134(12):e179742. doi: 10.1172/JCI179742. J Clin Invest. 2024. PMID: 39105625 Free PMC article. Review.
-
Myasthenogenicity of the main immunogenic region and endogenous muscle nicotinic acetylcholine receptors.Autoimmunity. 2012 May;45(3):245-52. doi: 10.3109/08916934.2011.622015. Epub 2011 Oct 21. Autoimmunity. 2012. PMID: 21950318 Free PMC article.
-
Myasthenogenicity of the main immunogenic region.Ann N Y Acad Sci. 2012 Dec;1274(1):9-13. doi: 10.1111/j.1749-6632.2012.06766.x. Ann N Y Acad Sci. 2012. PMID: 23252892 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

