Endothelial progenitor cell transplantation improves long-term stroke outcome in mice
- PMID: 20437584
- PMCID: PMC3026588
- DOI: 10.1002/ana.21919
Endothelial progenitor cell transplantation improves long-term stroke outcome in mice
Abstract
Objective: Endothelial progenitor cells (EPCs) play an important role in tissue repairing and regeneration in ischemic organs, including the brain. However, the cause of EPC migration and the function of EPCs after ischemia are unclear. In this study, we demonstrated the effects of EPCs on ischemic brain injury in a mouse model of transient middle cerebral artery occlusion (tMCAO).
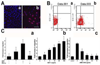
Methods: Circulating human EPCs were characterized with immunofluorescent staining and flow cytometry. EPCs (1 x 10(6)) were injected into nude mice after 1 hour of tMCAO. Histological analysis and behavioral tests were performed from day 0 to 28 days after tMCAO.
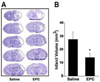
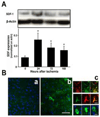
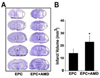
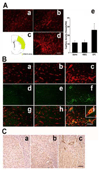
Results: EPCs were detected in ischemic brain regions 24 hours after tMCAO. EPC transplantation significantly reduced ischemic infarct volume at 3 days after tMCAO compared with control animals (p < 0.05). CXCR4 was expressed in the majority of EPCs, and stromal-derived factor-1 (SDF-1) induced EPC migration, which was blocked by pretreated EPCs with AMD3100 in vitro. SDF-1 was upregulated in ischemic brain. Compared with control animals, injecting AMD3100-pretreated EPCs resulted in a larger infarct volume 3 days after tMCAO, suggesting that SDF-1-mediated signaling was involved in EPC-mediated neuroprotection. In addition, EPC transplantation reduced mouse cortex atrophy 4 weeks after tMCAO and improved neurobehavioral outcomes (p < 0.05). EPC injection potently increased angiogenesis in the peri-infarction area (p < 0.05).
Interpretation: We conclude that systemic delivery of EPCs protects the brain against ischemic injury, promotes neurovascular repair, and improves long-term neurobehavioral outcomes. Our data suggest that SDF-1-mediated signaling plays a critical role in EPC-mediated neuroprotection.
Figures



References
-
- Miraglia S, Godfrey W, Yin AH, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. - PubMed
-
- Quirici N, Soligo D, Caneva L, et al. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–194. - PubMed
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. - PubMed
-
- Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

