Nucleophilic substitution of oxazino-/oxazolino-/benzoxazin [3,2-b]indazoles: an effective route to 1H-indazolones
- PMID: 20438102
- PMCID: PMC3108055
- DOI: 10.1021/ol100751n
Nucleophilic substitution of oxazino-/oxazolino-/benzoxazin [3,2-b]indazoles: an effective route to 1H-indazolones
Abstract
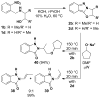
A variety of nucleophiles, thiolates, alkoxides, amines, iodide, and cyanide, react with oxazino-, oxazolino-, and benzoxazin[3,2-b]indazoles under microwave conditions to yield a diverse set of 2-substituted 1H-indazolones. The synthetic utility of these indazoles is further demonstrated by ANRORC (addition of the nucleophile, ring-opening, and ring closure) reactions to yield isomeric pyrazoloindazolones by a process wherein iodide acts first as a nucleophile and subsequently as a leaving group.
Figures





References
-
- Fletcher SR, Mclver E, Lewis S, Burkamp F, Leech C, Mason G, Boyce S, Morrison D, Richards G, Sutton K, Jones AB. Bioorg Med Chem Lett. 2006;16:2872. - PubMed
-
- Wang H, Han H, Von Hoff DD. Cancer Res. 2006;66:9722. - PubMed
-
- Kawanishi N, Sugimoto T, Shibata J, Nakamura K, Masutani K, Ikuta M, Hirai H. Bioorg Med Chem Lett. 2006;16:5122. - PubMed
-
- Abouzid KAM, El-Abhar HS. Arch Pharmacal Res. 2003;26:1. - PubMed
-
- Silvestrini B, Cheng CY. 99–304042 US Patent appl. 1999
- Cerecetto H, Gerpe A, Gonzalez M, Aran VJ, de Ocariz CO. Mini-Rev Med Chem. 2005;5:869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

