Activation of ASK-1 and downstream MAP kinases in cytochrome P4502E1 potentiated tumor necrosis factor alpha liver injury
- PMID: 20438834
- PMCID: PMC2900408
- DOI: 10.1016/j.freeradbiomed.2010.04.021
Activation of ASK-1 and downstream MAP kinases in cytochrome P4502E1 potentiated tumor necrosis factor alpha liver injury
Abstract
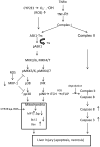
Cytochrome P4502E1 (CYP2E1) potentiates TNFalpha toxicity by a mechanism involving increased oxidative stress and activation of JNK and p38 MAPKs. This study evaluated the upstream mediators of this MAPK activation with a special focus on studying whether apoptosis signal regulating kinase-1 (ASK-1) is activated in the CYP2E1-TNFalpha hepatotoxic model. Wild-type and CYP2E1(-/-) mice were treated with pyrazole (PY) for 3days to induce CYP2E1 and challenged with TNFalpha on day 3. Liver injury occurred between 8 and 12h after TNFalpha administration only to the wild-type PY-treated mice. Oxidative stress was elevated in the PY mice at 4h, a time before the liver injury. ASK-1 was dissociated from the thioredoxin-ASK-1 complex and was activated at 4h after administration of TNFalpha to PY mice. This was followed by activation of MKK3/MKK6 and MKK4/MKK7 at 4-8 or 12h and then JNK/p38 MAPK at 8 to 12h. MAPK phosphatase-1 was decreased 12 to 24h after TNFalpha administration. This may promote a sustained activation of JNK. Bax was elevated, whereas Bcl-2 and cFLIP(S/L) were lowered at 4h after administration of TNFalpha. These changes were followed by increases in caspase 8 and 3 activities and apoptosis. None of the above changes were observed when TNFalpha was administered to PY-treated CYP2E1(-/-) mice. These studies show that TNFalpha increases oxidative stress in mice with elevated CYP2E1, with subsequent activation of ASK-1 via a mechanism involving thioredoxin-ASK-1 dissociation, followed by activation of downstream MKK and MAPK. We speculate that similar interactions between CYP2E1 and TNFalpha may be important for alcohol-induced liver injury.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures










References
-
- Thurman GR. Mechanisms of hepatic toxicity. II Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. - PubMed
-
- McClain CJ, Barve S, Deaciu I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–219. - PubMed
-
- Yin M, Wheeler MD, Kono H, Bradford BU, Galluci RM, Luster MI, Thurman RG. Essential role of TNFα in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. - PubMed
-
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. - PubMed
-
- Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Kadiisda M, Mason RP. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13:S39–S50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

