Regulation of coat assembly--sorting things out at the ER
- PMID: 20439155
- PMCID: PMC2910129
- DOI: 10.1016/j.ceb.2010.04.003
Regulation of coat assembly--sorting things out at the ER
Abstract
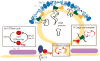
The small GTPase Sar1 resides at the core of a regulatory cycle that controls protein export from the ER in COPII vesicles. Recent advances in minimally reconstituted systems indicate continual flux of Sar1 through GTPase cycles facilitates cargo concentration into forming vesicles that ultimately bud from membranes. During export from ER membranes, this GTPase cycle is harnessed through the combinatorial power of multiple coat subunits and cargo adaptors to sort an expanding array of proteins into ER-derived vesicles. The COPII budding machinery is further organized into higher-order structures at transitional zones on the ER surface where the large multi-domain Sec16 protein appears to perform a central function.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. - PubMed
-
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. - PubMed
-
- Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

