Protein kinase C-beta gene variants, pathway activation, and enzastaurin activity in lung cancer
- PMID: 20439192
- PMCID: PMC3171168
- DOI: 10.3816/CLC.2010.n.021
Protein kinase C-beta gene variants, pathway activation, and enzastaurin activity in lung cancer
Abstract
Background: Protein kinase C-beta2 (PKCbeta2) is a splice-variant of the PRKCB1 gene and belongs to a family of serine/threonine-specific kinases that are predominantly activated by diacylglycerol, calcium, and phorbol ester. Cellular functions associated with PKCbeta2 activation include transformation, proliferation, and inhibition of apoptosis. Enzastaurin (LY317615) is an oral, selective, potent inhibitor of the PKCbeta2 kinase. Preclinical activity for this agent was predominantly reported in lymphoma, glioblastoma, and colorectal cancer. In patients with advanced non-small-cell lung cancer (NSCLC) whose previous therapy had failed, 13% of patients had disease control for 6 months with single-agent therapy.
Patients and methods: We investigated whether biologically relevant variants of PRKCB1 exist in lung cancer cell lines in the context of enzastaurin-induced proliferation and kinase inhibition, using exon sequencing, immunoblotting, and cytotoxicity assays in NSCLC and small-cell lung cancer (SCLC) cell lines.
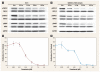
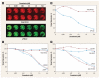
Results: We discovered a total of 6 single-nucleotide variants, but only 1 resulted in an amino acid substitution (T40I). This substitution was not located in the kinase domain of PKCbeta2 and did not affect enzastaurin's antiproliferative or phosphorylation-inhibitory activity. We found enzastaurin to be equally active in NSCLC and SCLC cell lines, with values of the 50% inhibitory concentration in a range of 0.05-0.2 microM.
Conclusion: The inhibition of phosphorylation of PKCbeta2 and the downstream molecules glycogen synthase kinase-3beta, S6RP, Akt, and forkhead transcription factor was evident in the same concentration range, which suggests the premise that the determination of phosphorylation levels of these molecules in human tissue specimens may be a useful pharmacodynamic parameter for in vivo target inhibition by enzastaurin.
Figures
References
-
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–94. - PubMed
-
- Barr L, Campbell S, Baylin S. Protein kinase C-beta 2 inhibits cycling and decreases c-myc-induced apoptosis in small cell lung cancer cells. Cell Growth Differ. 1997;8:381–92. - PubMed
-
- Clark A, West K, Blumberg P, et al. Altered protein kinase C (PKC) isoforms in non-small cell lung cancer cells: PKC-delta promotes cellular survival and chemotherapeutic resistance. Cancer Res. 2003;63:780–6. - PubMed
-
- Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase C-alpha in endothelial cells. Cell Signal. 2004;16:951–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

